April 15, 2016 report
Nuclear pore complex successfully mapped and diagramed
(Phys.org)—Two teams of researchers taking different approaches have successfully mapped and diagramed the nuclear pore complex (NPC)—protein complexes that make up the pores in the nuclear envelope that allow or prevent passage of molecules into the nucleus. One team from the California Institute of Technology took a bottom-up approach to discovering the makeup of the pore rings, while a team with members from several institutions in the U.S. and Germany took a top-down approach. Both have published their results in the journal Science.
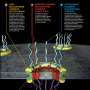
In order to carry out its various functions, the nucleus in every cell must be able to receive certain molecules and send others to other parts of the cell, but because other unnecessary or dangerous molecules can make their way into the cell, a means of protecting the nucleus has evolved—a double membrane which serves as a barrier. That barrier has on average, 2000 pores in it, each of which serve as a gatekeeper, either allowing a molecule through, or barring its entry. Though the job is very important, because it serves as a means of stopping viral or bacterial attacks, its structure has not been very well understood, until now. In these two new efforts, both teams have managed to create maps of the different layers that make up the rings of the pores, offering other medical researchers a new way perhaps, to combat diseases that manage to make it through the NPC.
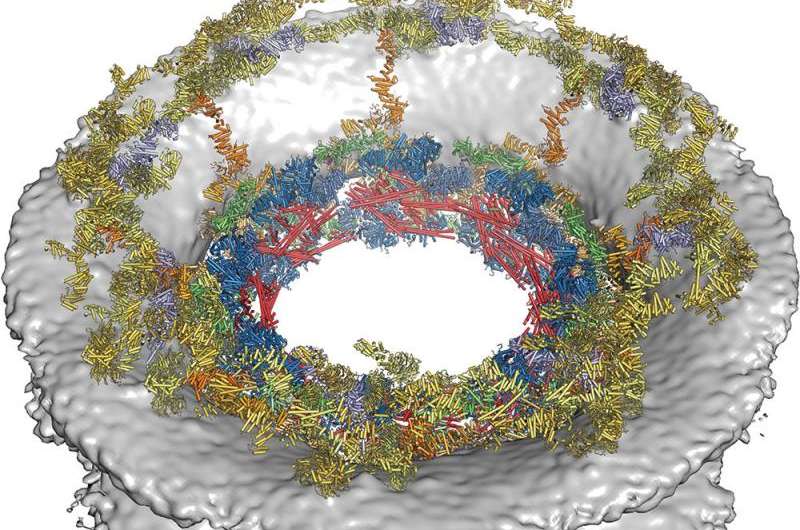
The team at Caltech has been working on the problem for 10 years, using electron cryotomography to study each part of each ring, successively adding more and more to a growing map. The end result describes all of the interconnections and interactions of all of the proteins in each pore, which is itself part of a larger system. The second team took the opposite approach, using mass spectrometry, cryo-electron microscopy and finally computer modeling to create a realistic rendering of an actual pore.
As both teams note, now that the structure of the NPC has finally been mapped, the next step will be to learn more about how all the pieces work together to control the flow of molecules in and out of the nuclear envelope and perhaps to devise a means for providing assistance when the NPC fails.
More information: D. H. Lin et al. Architecture of the symmetric core of the nuclear pore, Science (2016). DOI: 10.1126/science.aaf1015
Abstract
The nuclear pore complex (NPC) controls the transport of macromolecules between the nucleus and cytoplasm, but its molecular architecture has thus far remained poorly defined. We biochemically reconstituted NPC core protomers and elucidated the underlying protein-protein interaction network. Flexible linker sequences, rather than interactions between the structured core scaffold nucleoporins, mediate the assembly of the inner ring complex and its attachment to the NPC coat. X-ray crystallographic analysis of these scaffold nucleoporins revealed the molecular details of their interactions with the flexible linker sequences and enabled construction of full-length atomic structures. By docking these structures into the cryoelectron tomographic reconstruction of the intact human NPC and validating their placement with our nucleoporin interactome, we built a composite structure of the NPC symmetric core that contains ~320,000 residues and accounts for ~56 megadaltons of the NPC's structured mass. Our approach provides a paradigm for the structure determination of similarly complex macromolecular assemblies.
J. Kosinski et al. Molecular architecture of the inner ring scaffold of the human nuclear pore complex, Science (2016). DOI: 10.1126/science.aaf0643
Abstract
Nuclear pore complexes (NPCs) are 110-megadalton assemblies that mediate nucleocytoplasmic transport. NPCs are built from multiple copies of ~30 different nucleoporins, and understanding how these nucleoporins assemble into the NPC scaffold imposes a formidable challenge. Recently, it has been shown how the Y complex, a prominent NPC module, forms the outer rings of the nuclear pore. However, the organization of the inner ring has remained unknown until now. We used molecular modeling combined with cross-linking mass spectrometry and cryo-electron tomography to obtain a composite structure of the inner ring. This architectural map explains the vast majority of the electron density of the scaffold. We conclude that despite obvious differences in morphology and composition, the higher-order structure of the inner and outer rings is unexpectedly similar.
Journal information: Science
© 2016 Phys.org