Nanoporous material's strange 'breathing' behavior
High-tech sponges of the infinitely small, nanoporous materials can capture and release gaseous or liquid chemicals in a controlled way. A team of French and German researchers from the Institut de Recherche de Chimie Paris (CNRS/Chimie ParisTech) and the Institut Charles Gerhardt de Montpellier (CNRS/Université de Montpellier/ENSCM) has developed and described one of these materials, DUT-49, whose behavior is totally counterintuitive. When pressure is increased for a sample of DUT-49 to absorb more gas, the material contracts suddenly and releases its contents—as if, when inhaling, the lungs contracted and expelled the air that they contained. This work, published in Nature on April 6, 2016, makes it possible to envisage innovative behavior in materials science.
Capturing toxic molecules in ambient air, storing hydrogen, targeting drug release—the list of applications that could use flexible nanoporous materials is endless. These materials use the large surface area in their pores to capture and store gaseous or liquid molecules: this phenomenon is called adsorption. Their pores can adsorb impressive quantities of products; they keep getting bigger until they reach their flexibility limit.
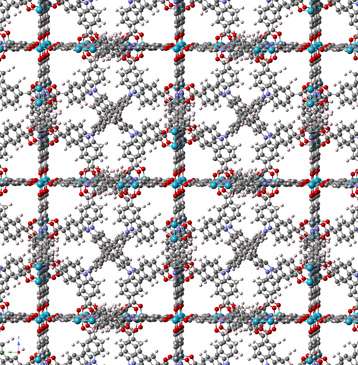
A French and German team has designed a novel type of nanoporous material: DUT-49. Formed by self-assembly from a carbon skeleton and copper atoms, its structure is both organic and metallic. It is an incredibly porous powder: the internal surface area of a single gram of this material is 5,000 m². So DUT-49 can adsorb the equivalent of a third of its weight in methane. Like other "intelligent" materials in its family, its properties change based upon external stimulations, such as pressure, temperature or light. If the pressure is increased while a gas is being captured, both the amount of gas adsorbed and, for the most common case, the material's pore size increase. DUT-49's unusually high flexibility causes an unexpected phenomenon though: as the material fills with gas, it contracts suddenly when it reaches a certain pressure and its volume halves if the pressure continues to increase.

Researchers originally regarded this an instrumental failure, because none of the millions of other known materials that adsorb gases behave in this way. However this "negative adsorption" phenomenon has been confirmed by extra measurements and the team has been able to describe the mechanism. The gas molecules stored in the pores of DUT-49 establish strong interactions with the solid's structure. Depending on the amount of gas adsorbed, this can disturb the arrangement of the atoms that form the material and eventually cause it to contract. This specific behavior has been tested with butane and methane and is expected to be applicable to other gaseous compounds in DUT-49.
DUT-49 is another recently discovered material with "abnormal" physical properties, such as those with negative thermal expansion that contract when they are heated. This result opens up a broad field of study on flexible porous materials and their innovative behavior in materials science. This could lead to developing nanometric switches and sensors. The material's deflation is a strong response to a small event triggered from an easily detected threshold value.
More information: Simon Krause et al. A pressure-amplifying framework material with negative gas adsorption transitions, Nature (2016). DOI: 10.1038/nature17430
Journal information: Nature
Provided by CNRS