Exotic quantum effects can govern the chemistry around us
Objects of the quantum world have a concealed and cold-blooded nature—they usually behave in a quantum manner only when they are significantly cooled and isolated from the environment. Experiments carried out by chemists and physicists from Warsaw have changed this simple picture. It turns out that not only does one of the most interesting quantum effects occur at room temperature and higher, but it plays a dominant role in the course of chemical reactions in solutions.
We generally derive our experimental knowledge of quantum phenomena from experiments carried out using sophisticated equipment under exotic conditions: at extremely low temperatures and in a vacuum, isolating quantum objects from the disturbing influence of the environment. Scientists from the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw, led by Prof. Jacek Waluk and Prof. Czeslaw Radzewicz's group from the Faculty of Physics, University of Warsaw (FUW), have just shown that one of the most spectacular quantum phenomena—quantum tunneling—takes place even at temperatures above the boiling point of water. However, what is particularly surprising is the fact that the observed effect applies to hydrogen nuclei, which tunnel in particles floating in solution. The measurements leave no doubt that in conditions typical for our environment, tunneling turns out to be the main factor responsible for the chemical reaction.
"For some time, chemists have been getting used to the idea that electrons in molecules can tunnel. We have shown that in the molecule, it is also possible for protons—that is, nuclei of hydrogen atoms—to tunnel. So we have proof that a basic chemical reaction can occur as a result of tunneling in solution and at room temperature or higher," explains Prof. Waluk.
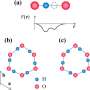
In their experiments, the Warsaw researchers studied single molecules of porphycene (C20H14N4), an isomer of porphyrin. Compounds belonging to this group occur naturally, for example in human blood, where they are involved in the transport of oxygen. Their molecules are in the form of planar carbon rings with hydrogen atoms outside and four nitrogen atoms inside, arranged at the corners of a tetragon. In the space surrounded by nitrogen atoms there are two protons. These protons can move between the nitrogen atoms. The open question was whether they do so by moving classically or by tunneling.
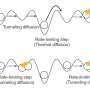
Tunneling is a consequence of the probabilistic nature of quantum objects. In the classical world known to us from everyday life, an object will always with total probability be in one place, and therefore with zero probability be in all others. Not so in the quantum world. When nothing disturbs the state of an elementary particle, atom or a small group of them, the probability of the existence of a quantum object dissolves in space. This phenomenon leads to spectacular effects. When a man wants to surmount a wall, he has to climb it—that is, he has to increase his gravitational energy until it becomes greater than the potential barrier set by the wall. Meanwhile, the indeterminacy of the quantum object means that it can be found on the other side of the barrier, without increasing its energy—simply 'passing through.' The effect occurs much faster than ordinary transfer in space and with a probability that is greater the smaller the distance over which the object tunnels. By studying the times of the proton's jumps, it can be determined if they have moved classically or if they have tunneled.
"Reality is less clear-cut. The higher our proton climbs the energy ladder of porphycene, the smaller the width of the barrier to overcome. Tunneling then becomes increasingly likely. So everything indicates that before the proton has time to climb to an energy level allowing it to classically overcome the potential barrier, it has usually tunneled anyway," explains Prof. Waluk.
Climbing the potential barrier is not simple. When we supply the protons in porphycene with energy, we also induce vibrations in the molecule itself. It turns out that among 108 possible modes of vibration in a molecule of porphycene, some increase the probability of tunneling and others decrease it. The Warsaw-based researchers, funded by grants from the Polish National Science Centre, determined the rate constants of chemical reactions involving porphycene in the temperature range from 20 to 400 Kelvin for proton jumps occurring in the lowest energy state of the molecule, and in one of the excited vibrational states, promoting tunneling. The timing of proton jumps between the nitrogen atoms were thus obtained. Experiments conducted on sets of cold, isolated particles suggested times of a few picoseconds (a millionth of one millionth of a second), and such times were observed in experiments in Warsaw, led by Dr. Piotr Fita and Ph.D. student Piotr Ciacka from the FUW. Measurements show that not only does tunneling occur in porphycene, but it is responsible, even at room temperature, for at least 80 percent of the proton jumps in the centres of the molecules.
The dominant role of tunneling in the course of a chemical reaction and its dependence on the type of vibration of the molecule is a means to incredibly precise control of the course of chemical reactions. This sort of chemistry, known as mode-selective chemistry, has been demonstrated previously, but at a very low temperature. The discovery of the researchers from the IPC PAS and the FUW suggests that in the future, it will be possible to accurately control reactions taking place under conditions typical for our environment. Chemical molecules floating in solution, previously excited in a manner that enhances their reactivity, could be introduced into a state of oscillation that significantly reduces their reactivity (or vice versa). A specific reaction, perhaps one of many taking place in the solution, could then be switched on and off on demand, by small changes in the amount of energy supplied to the molecules of a selected compound.
"The tunneling of protons in molecules of porphycene in solution is spectacular proof that even at room temperature and in a dense environment, a purely quantum effect can rule the course of a chemical reaction. But this is not the end of the surprises. We have a reasonable suspicion that one more exotic quantum phenomenon is involved in the movements of the two protons in porphycene, always jumping together. The world of chemistry around us would then be even more interesting," says Prof. Waluk.
More information: Piotr Ciąćka et al. Evidence for Dominant Role of Tunneling in Condensed Phases and at High Temperatures: Double Hydrogen Transfer in Porphycenes, The Journal of Physical Chemistry Letters (2016). DOI: 10.1021/acs.jpclett.5b02482
Journal information: Journal of Physical Chemistry Letters
Provided by Polish Academy of Sciences