Superman can start worrying—we've almost got the formula for kryptonite
Theoretical chemists from the Institute of Physical Chemistry of the Polish Academy of Sciences have discovered how to synthesize a krypton oxide, the first binary compound of krypton and oxygen. It turns out that this exotic substance can be produced under extremely high pressure, and its production is within the capabilities of today's laboratories.
Crystals of kryptonite, a material deadly to Superman and his race, were supposed to have been created within the planet Krypton, most likely under very high pressure. The planet's name is derived from the element krypton, with the atomic number of 36, a noble gas considered to be incapable of forming stable chemical compounds. However, a publication in the journal Scientific Reports by a two-man team of theoretical chemists from the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw, Poland, predict the synthesis of a new crystalline material in which atoms of krypton would be chemically bonded to another element.
"The substance we are predicting is a compound of krypton with oxygen, not nitrogen. In the convention of the comic book it should, therefore, be called 'kryptoxide,' not 'kryptonite.' So if Superman's reading this, he can stay calm—at the moment, there's no cause for panic," says Dr. Patrick Zaleski-Ejgierd (IPC PAS). "Our krypton monoxide, KrO, probably does not exist in nature. According to current knowledge, the deep interiors of planets are the only place where there is sufficient pressure for its synthesis. Oxygen does not exist there, nor does krypton."
Compounds of krypton have been produced in the laboratory under cryogenic conditions. They were, however, only single, linear and small molecules of the hydrogen-carbon-krypton-carbon-hydrogen type. The Polish chemists wondered if there were conditions in which krypton would not only bond chemically with another element, but also in which it would be capable of forming an extensive and stable crystal lattice. Their search, funded by an OPUS grant from the Polish National Science Centre, involved using genetic algorithms and models built on the so-called density functional theory. In the field of solid-state physics, this theory is a basic tool for the description and study of the world of chemical molecules.
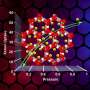
"Our computer simulations suggest that crystals of krypton monoxide will be formed at a pressure in the range of 300 to 500 million atmospheres. This is a high pressure, but it can be achieved even in today's laboratories, by skillfully squeezing samples in diamond anvils," says Ph.D. student Pawel M. Lata (IPC PAS).
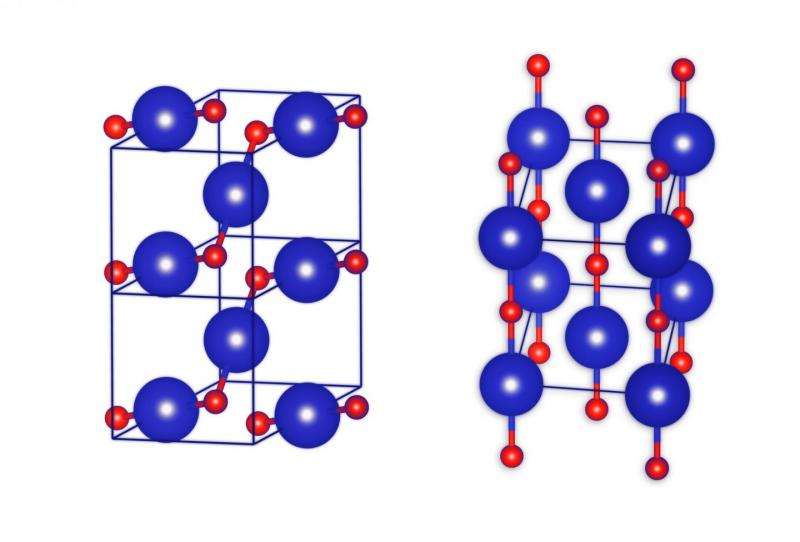
Crystal lattices are built from atoms or molecules arranged in space in an orderly manner. The smallest repetitive fragment of such structures, the basic 'building block,' is called a unit cell. In crystals of table salt, the unit cell has the shape of a cube in which the sodium and chlorine atoms, arranged alternately, are mounted on each corner, close enough to each other that they are bound by covalent (chemical) bonds.
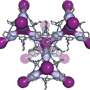
The unit cell of krypton monoxide is cuboid with a diamond base, with krypton atoms at the corners. In addition, in the middle of the two opposite side walls, there is one atom of krypton.
"Where is the oxygen? On the side walls of the unit cell, where there are five atoms of krypton, they are arranged like the dots on a dice showing the number five. Single atoms of oxygen are located between the krypton atoms, but only along the diagonal—and only along one. Thus, on each wall with five krypton atoms, there are only two atoms of oxygen. Not only that, the oxygen is not exactly on the diagonal: One of the atoms is slightly offset from it in one direction and the other atom in the other direction," says Lata.
In such an idiosyncratic unit cell, each atom of oxygen is chemically bound to the two nearest adjacent atoms of krypton. Zigzag chains of Kr/OKrO/Kr will therefore pass through the crystal of krypton monoxide, forming long polymer structures. Calculations indicate that crystals of this type of krypton monoxide should have the characteristics of a semiconductor. One can assume that they will be dark, and their transparency will not be great.
Theorists from the IPC PAS have also found a second, slightly less stable compound of krypton: the tetroxide KrO4. This material, which probably has properties typical of a metal, has a simpler crystalline structure and could be formed at a pressure exceeding 340 million atmospheres.
After formation, the two kinds of krypton oxide crystals could probably exist at a somewhat lower pressure than that required for their formation. The pressure on earth, however, is so low that on our planet these crystals would undergo degradation immediately.
"Reactions occurring at extremely high pressure are almost unknown, very, very exotic chemistry. We call it 'Chemistry on the Edge.' Often, the pressures needed to perform syntheses are so gigantic that at present, there is no point in trying to produce them in laboratories. In those cases, even methods of theoretic description fail! But what is most interesting here is the non-intuitiveness. From the very first to the last step of synthesis you never know what's going to happen," says Dr. Zaleski-Ejgierd.
More information: Patryk Zaleski-Ejgierd et al. Krypton oxides under pressure, Scientific Reports (2016). DOI: 10.1038/srep18938
Journal information: Scientific Reports
Provided by Polish Academy of Sciences