Scientists find the temperature at which glass becomes a liquid
While glass might be thought of in terms of holding wine or as a window, the stability of glass affects areas as diverse as nuclear waste storage, pharmaceuticals, and ice cream. Recently, chemical physicists at Pacific Northwest National Laboratory made a key discovery about how glass forms.
They discovered that the temperature at which glass-forming materials are deposited on a substrate affects the stability. Their findings, published in The Journal of Physical Chemistry Letters, show the ability of a technique called inert gas permeation to tell at what temperature a solid "melts." Their work brings more understanding to the fundamental properties of glass.
"Glasses are metastable materials with the mechanical properties of a solid-you can touch and hold them, versus a gas," said Dr. Scott Smith, a co-author on the paper. "But they are not like crystalline materials, which are in a perfect array. The molecules in glasses are arranged in a disordered pattern. In liquids the molecules are constantly moving, if you suddenly freeze a liquid, the molecules are randomly oriented and unstructured. In some sense, a glass can be thought of as a frozen liquid."
No matter how glass is made, understanding its properties is important. For example, the reason some medications have expiration dates is that their physical state changes from amorphous to crystalline. Once that happens, the medication doesn't dissolve as readily when taken and is thus ineffective. Finding ways to increase its stability and effectiveness would extend its shelf life. Similarly, when nuclear waste is put into a glass matrix, the glass must remain stable to keep the radionuclides from being released. And as most ice cream lovers know, when you open a carton and see crystals have formed on the surface, it has lost much of its flavor.
"Our research is fundamental work that could be important for stable glass manufacture by adding to understanding of liquids and liquid behavior," Smith said. Glasses depend on temperature for stability. At the correct temperature, a glass remains stable because its molecules stay put. At warmer temperatures, it transforms into a supercooled liquid and then crystallizes.
To create a glass, the materials must be cooled rapidly to a temperature low enough that the molecules don't have enough time or energy to find the lowest energy configuration (a crystal). That temperature is called the glass transition temperature, or Tg, and it varies depending on the experimental conditions and the cooling rate.
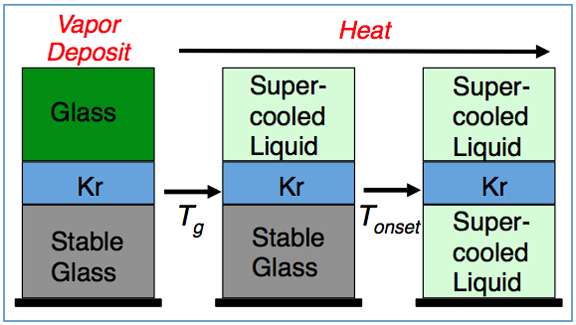
Smith and colleagues Dr. Alan May and Dr. Bruce Kay took the glass-forming materials toluene and ethylbenzene and super cooled them by depositing them onto a surface at 30 K. When the materials hit the surface, they formed an amorphous solid—a glass. The researchers then heated the sample. A layer of krypton deposited between two layers of glassy material (a sandwich) remained trapped until the glass transformed into a supercooled liquid (see Figure). The onset of gas release revealed at what temperature the glass transformed into a supercooled liquid.
The researchers varied the material deposition temperature from 40 to 130 K. They observed that the stability of the glass depended on the deposition temperature. They found that for both toluene and ethylbenzene, deposition at a temperature a few degrees less than Tg, created the most stable glass-one that was the most resistant to turning into a supercooled liquid. These results are consistent with the calorimetric studies of Prof. Mark Ediger at the University of Wisconsin-Madison.
"We found we can control one variable: deposition temperature. Even a difference of one Kelvin can result in years of difference in material lifetime and stability," said Smith.
More information: R. Scott Smith et al. Probing Toluene and Ethylbenzene Stable Glass Formation Using Inert Gas Permeation, The Journal of Physical Chemistry Letters (2015). DOI: 10.1021/acs.jpclett.5b01611
Journal information: Journal of Physical Chemistry Letters
Provided by Pacific Northwest National Laboratory




















