Scientists control chemical reactions with static electricity
Scientists have harnessed static electricity to control chemical reactions for the first time, in a breakthrough that could bring cleaner industry and cheaper nanotechnology.
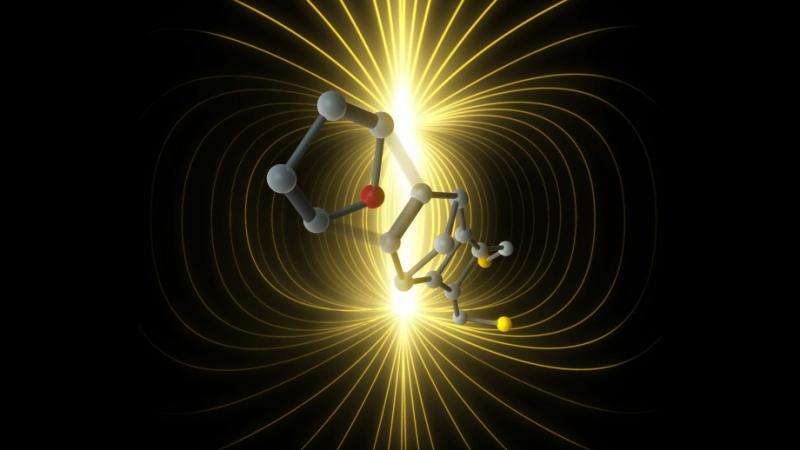
The team used an electric field as a catalyst for a common reaction, the Diels-Alder reaction, improving its reaction rate by a factor of five.
Lead researcher Professor Michelle Coote from ANU Research School of Chemistry said the team had overturned conventional thinking with their new-found control of the common reaction, which is used to make a range of chemicals from self-healing materials to the drug cortisone.
"It's the most unexpected result possible," said Professor Coote, who is also a Chief Investigator with ARC Centre of Excellence for Electromaterials Science (ACES). "We now have a totally new way of thinking about chemistry.
"The breakthrough could speed up manufacturing processes and allow unprecedented control of chemical reactions, for example in manufacturing flexible electronic components based on organic circuits," she said.
An electric field catalyst is completely different to conventional catalysts, which are often based on expensive rare chemicals and can create unwanted by-products or contaminate the final products.

As electric fields can be turned on and off very quickly from outside the test-tube, the new approach gives the researchers remote control over chemical processes.
Professor Coote predicted that electric fields could strongly affect reaction rates, but it had never been observed before because standard chemical reactions are conducted with molecules oriented in random directions in a gas or liquid.
The Centre of Excellence brought together a team, including researchers from Universitat de Barcelona in Spain and Dr Simone Ciampi from the University of Wollongong, that devised a way to test Professor Coote's prediction, using the electric field generated by the tip of a scanning-tunneling electron microscope.
The group oriented all the molecules in the same direction by attaching them to a surface and then used the probe of the electron microscope to test each molecule.
By changing the strength and polarity of the electric field, the team were able to vary the rate of the Diels-Alder reaction, in which a conjugated diene and a substituted diene form a cyclohexene system, by a factor of five
Professor Coote said the result will help research understand a lot of natural biochemical reactions.
"Nature uses enzymes as the ultimate catalyst, which can vary reaction rates by 14 orders of magnitude," she said.
"Enzymes work with carefully oriented charged functional groups, held in precise orientations, effectively generating an oriented electric field within the active site."
Professor Coote started the project when ANU joined the University of Wollongong as an ACES partner and was introduced to colleagues at Universitat de Barcelona.
ACES Director Professor Gordon Wallace said it is only a holistic approach to interdisciplinary research that can take ideas to industries in the shortest time possible.
"The collegial environment and multidisciplinary approach within ACES meant that we could mobilise all the skills necessary-from molecular modelling to exquisite experiments-very quickly to enable this amazing discovery," Professor Wallace said.
The breakthrough has been published in the latest edition of Nature.
More information: Albert C. Aragonès et al. Electrostatic catalysis of a Diels–Alder reaction, Nature (2016). DOI: 10.1038/nature16989
Journal information: Nature
Provided by Australian National University