The air-water interface, when linked to capillarity, influences water retention or evaporation
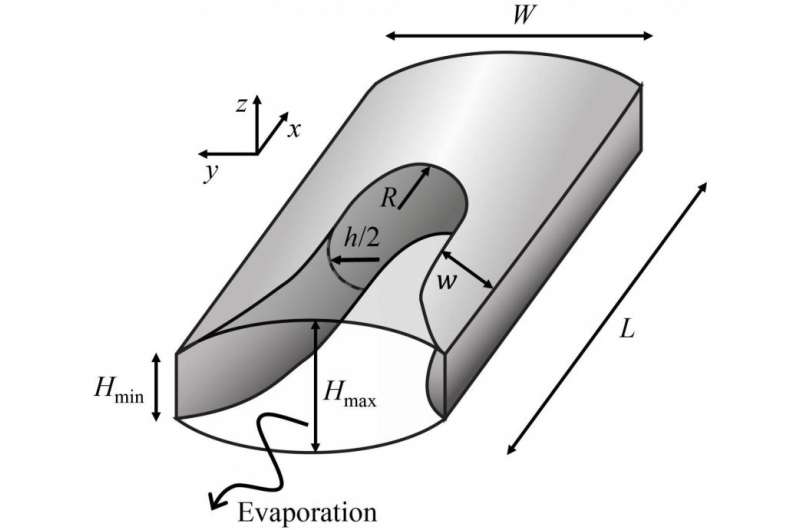
Water in, water out: such is the cycle of porous material. In some cases, like with soils, it is preferable to keep water in. In others, it makes better economic and ecological sense to have porous materials dry faster, e.g. in the paper industries or with plasterboard manufacturing. Modeling how porous material retains water or dries up can be resolved by narrowing the focus down to a single porous channel; now, a team of physicists has uncovered subtle underlying effects. These include the local shape of the air and water interface, which, in turn, is influenced by the actual shape of the capillaries. Emmanuel Keita, a physicist from Paris-Est University, France, who is also affiliated with Harvard University, Massachusetts, USA, and colleagues have just published these results in EPJ E .
The drying or water-retention process cannot typically be observed directly due to the opacity of the material. In this study, the authors relied on a simple glass channel which is thin enough to reveal dominant capillary effects—and not just effects linked to waters' fluid nature—that make the water travel along the channel. Using direct microscopic images, they observed the entire air-water interface throughout the capillary, which they then combined with ultra-precise mass measurements and numerical simulations.
The traditional assumption has been that drying is faster at the surface, since evaporation progresses from the surface down into the depth of the materials. However, this approach did not account for capillary pressure triggering water to move within pores. This study's simulations show that the drying rate changes according to the shape of the air-water interface located near the surface. This implies that slight changes to the wetting conditions near the surface can significantly modify the drying rate. Improving drying time—or indeed water retention capabilities—could therefore be achieved by controlling the porous materials' microstructure.
More information: Emmanuel Keita et al. Drying kinetics driven by the shape of the air/water interface in a capillary channel, The European Physical Journal E (2016). DOI: 10.1140/epje/i2016-16023-8
Journal information: European Physical Journal E
Provided by SciencePOD


















