How a novel technique could help combat antibiotic resistance
Antibiotic resistance is one of the greatest challenges facing global public health, threatening our ability to treat common infections. Cutting-edge research in Oxford University's Department of Chemistry is exploring new ways of administering antibacterial vaccines that could combat growing resistance from bacteria.
Dr Lingbing Kong is the joint first author (with Dr Balakumar Vijayakrishnan) of a new study, published in Nature Chemistry, exploring a method of vaccination that could circumvent existing resistance pathways by inhibiting, rather than killing, certain species of bacteria. Dr Kong, a member of Professor Ben Davis's research group when the study was carried out, told Science Blog how it all works.
Tell us about the background to your work on antibacterial vaccines
We first started looking at non-toxic antibacterials in 2007. We believed that they might better combat bacterial drug resistance since they do not kill the sensitive bacteria but merely increase their vulnerability in humans. We made our first step through the discovery of the first inhibitor of the capsular polysaccharide (CPS) transporter called Wza, which is common among Gram-negative bacteria. This is the foundation of our antibacterial strategy, which we also call the 'vaccination+block' approach. A report of this early work, carried out under the supervision of Professor Ben Davis and Professor Hagan Bayley, was published in Nature Chemistry in 2013.
How does this potential new vaccine work?
We have been working in collaboration with GlycoVaxyn (now LimmaTech and GSK). The vaccine candidate we developed is targeting a carbohydrate molecule that is common among most Gram-negative bacteria, such as notorious pathogenic bacteria Neisseria meningitidis, Pseudomonas aeruginosa and Escherichia coli. This molecule is a tetrasaccharide (four sugar units linked together) found at the base of a polysaccharide, known as a lipopolysaccharide (LPS). LPS is further covered by the outermost sugar layer, which is composed of CPS.
How could it combat antibiotic resistance?
In order to explain it further, we need to have a look at how the modern drug resistance problem occurred. Most current antibiotics kill bacteria through direct toxicity. There are two key mechanisms through which bacteria can then acquire resistance. In the first, mutations, which evolve over many generations, prevent the antibiotic from functioning. In the other, these mutations are acquired through gene transfer from neighbouring non-sensitive bacterial species.
Currently, the administration of an antibiotic results in the death of sensitive strains and the subsequent complete dominance of resistant strains within the habitat. Increased numbers of resistant bacteria accelerates the spread of resistant bacteria, and ultimately the arrival of multi-drug-resistant bacteria ('superbugs').
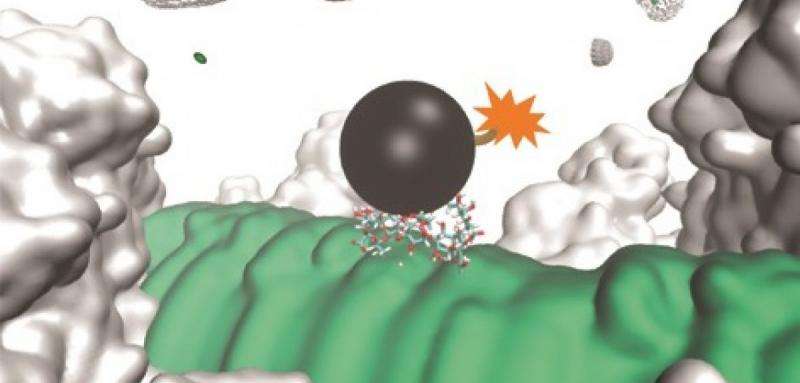
Our antibacterial strategy involves the administration of a non-toxic chemical (CPS inhibitor), which destroys the protective outermost CPS layer, exposing the bacteria to attacks by antibodies generated through vaccination. This is followed by cascade immune responses. Our strategy dramatically decreases the occurrence of antibiotic resistance by only killing the pathogenic bacteria within the human body and limiting the optimal effects to occur only after a combined use of vaccination and CPS inhibitor.
Which infections could it target?
We have demonstrated our strategy against infections caused by pathogenic E. coli, with potential against those caused by Neisseria meningitidis, Pseudomonas aeruginosa etc. We expect that our strategy could be extended to infections caused by most, if not all, Gram-negative bacterial pathogens since the CPS inhibitor is a general extracellular inhibitor. We have also demonstrated that after vaccination and upon administration of the CPS inhibitor, the first step of bacterial infections (attachment of bacteria to the host cells) could be prevented. This opens the possibility of rapid treatment of bacterial sepsis.
What are the next steps?
There are a few clear steps for moving forward. For example, we are currently trying to develop the vaccine candidates with larger carbohydrate molecules (more sugar units than the current four-unit molecule) for higher affinity and specificity towards the lipopolisaccharides in bacterial pathogens.
More information: Lingbing Kong et al. Single-molecule interrogation of a bacterial sugar transporter allows the discovery of an extracellular inhibitor, Nature Chemistry (2013). DOI: 10.1038/nchem.1695
Journal information: Nature Chemistry
Provided by University of Oxford