Shaping crystals with the flow

OIST scientists designed a new method to create crystals using a combination of shear flow and controlled temperature.
One of goals of the Okinawa Institute of Science and Technology Graduate University (OIST) is to foster collaboration between different disciplines. Recently, OIST scientists combined techniques from soft matter physics and structural biology to create and visualise crystals made of surfactants, surface-acting agents that are added in a multitude of products like detergents, cosmetics and paints. Crystals, high ordered arrangement of atoms in 3D, are sought after in both biology and physics, but are often very challenging to produce. This interdisciplinary study, published in Scientific Reports, can pave the way for faster drug discovery and delivery, and has several applications in the pharmaceutical, material sciences and biotechnology industry.
In this study, scientists used a combination of two biocompatible surfactants, one not-charged biocompatible surfactant called Tween80, often used in pharmaceutical formulations and cosmetics, and another one found in coconut oil, known as Monolaurin. Surfactants have a love-and-hate relationship with water: part of the surfactant molecule likes water and another part repels it. That is the reason why, surfactants dissolved in water tend to form micelles, which are spherically shaped aggregates with the water-friendly chemical groups positioned on the outside. The transition from spherical micelles to crystals is a quite complex process and goes through a series of shape changes. OIST scientists found that at the right temperature, the spherical micelles confined in a narrow space and rubbed by shear flow, organise into different shapes.
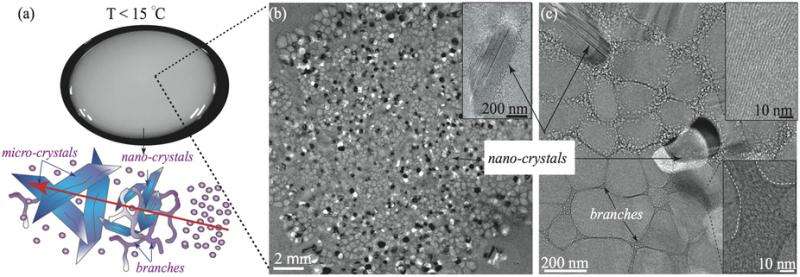
"After just 5 minutes, we saw that the samples became turbid and milky. We asked our colleagues of the Structural Cellular Biology unit to have a look at them and we found out that the milky solution contained worm-like micelles, branched micelles and crystal-like structures," describes Dr. Joshua Cardiel, former PhD student with Prof. Amy Shen, leader of OIST's Micro/Bio/Nanofluidics unit, and now a postdoctoral researcher at the National Institute of Standards and Technology (NIST) in the US.
OIST's Structural Cellular Biology unit, led by Prof. Ulf Skoglund, used a form of microscopy called cryogenic electron-microscopy (cryo-EM), where the sample is frozen and studied at temperatures close to minus 200°C. Cryo-EM photos show that the turbid appearance of the sample is due to the transition from spherical micelles into more ordered structures. Spherical micelles organise into wormlike micelles, then develop into branched micelles and finally transform into ordered crystal-like structures.
"Traditional crystallography is based on removing water from the sample chemically, so called 'salting out', usually associated with smaller temperature variations to spot optimal crystallization conditions. Our method took advantage of adding external shear flow, combined with ambient temperatures to induce controlled crystallization," explains Prof. Shen.
"Every crystallographer knows that obtaining a good crystal is a very long and challenging process. This technique has the potential to reproducibly create crystals that are only 0.1 µm, which is 100 times smaller than the current ones used in X-ray crystallographic studies," comments Prof. Skoglund. "We will continue to work together with physicists to explore the possibilities of this new method."
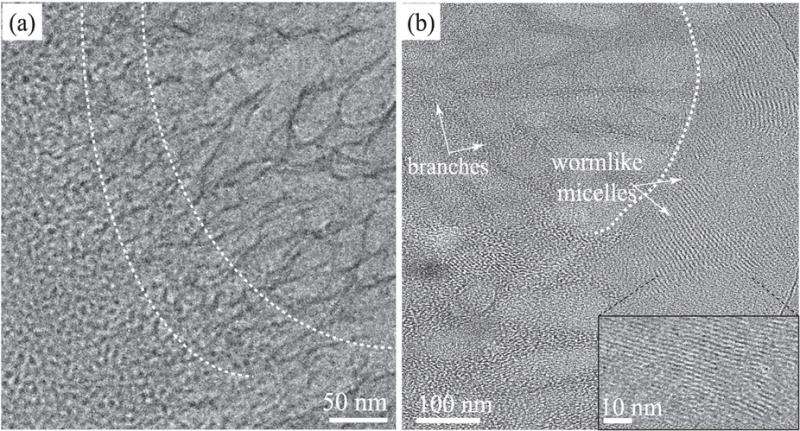
More information: Joshua J. Cardiel et al. Formation of crystal-like structures and branched networks from nonionic spherical micelles, Scientific Reports (2015). DOI: 10.1038/srep17941
Journal information: Scientific Reports
Provided by Okinawa Institute of Science and Technology