Battery technology could charge up water desalination

The technology that charges batteries for electronic devices could provide fresh water from salty seas, says a new study by University of Illinois engineers. Electricity running through a salt water-filled battery draws the salt ions out of the water.
Illinois mechanical science and engineering professor Kyle Smith and graduate student Rylan Dmello published their work in the Journal of the Electrochemical Society.
"We are developing a device that will use the materials in batteries to take salt out of water with the smallest amount of energy that we can," Smith said. "One thing I'm excited about is that by publishing this paper, we're introducing a new type of device to the battery community and to the desalination community."
Interest in water desalination technology has risen as water needs have grown, particularly in drought-stricken areas. However, technical hurdles and the enormous amounts of energy required have prevented wide-scale implementation. The most-used method, reverse osmosis, pushes water through a membrane that keeps out the salt, a costly and energy-intensive process. By contrast, the battery method uses electricity to draw charged salt ions out of the water.
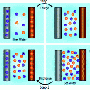
The researchers were inspired by sodium ion batteries, which contain salt water. Batteries have two chambers, a positive electrode and a negative electrode, with a separator in between that the ions can flow across. When the battery discharges, the sodium and chloride ions - the two elements of salt - are drawn to one chamber, leaving desalinated water in the other.
In a normal battery, the ions diffuse back when the current flows the other direction. The Illinois researchers had to find a way to keep the salt out of the now-pure water.
"In a conventional battery, the separator allows salt to diffuse from the positive electrode into the negative electrode," Smith said. "That limits how much salt depletion can occur. We put a membrane that blocks sodium between the two electrodes, so we could keep it out of the side that's desalinated."
See a video of how it works on YouTube at https://www.youtube.com/watch?v=3QWoEOAlOzM&feature=youtu.be.
The battery approach holds several advantages over reverse osmosis. The battery device can be small or large, adapting to different applications, while reverse osmosis plants must be very large to be efficient and cost effective, Smith said. The pressure required to pump the water through is much less, since it's simply flowing the water over the electrodes instead of forcing it through a membrane. This translates to much smaller energy needs, close to the very minimum required by nature, which in turn translates to lower costs. In addition, the rate of water flowing through it can be adjusted more easily than other types of desalination technologies that require more complex plumbing.
Smith and Dmello conducted a modeling study to see how their device might perform with salt concentrations as high as seawater, and found that it could recover an estimated 80 percent of desalinated water. Their simulations don't account for other contaminants in the water, however, so they are working toward running experiments with real seawater.
"We believe there's a lot of promise," Smith said. "There's a lot of work that's gone on in developing new materials for sodium ion batteries. We hope our work could spur researchers in that area to investigate new materials for desalination. We're excited to see what kind of doors this might open."
More information: Kyle C. Smith et al. Na-Ion Desalination (NID) Enabled by Na-Blocking Membranes and Symmetric Na-Intercalation: Porous-Electrode Modeling, Journal of The Electrochemical Society (2016). DOI: 10.1149/2.0761603jes
Provided by University of Illinois at Urbana-Champaign