A molecular light switch?... Just add water
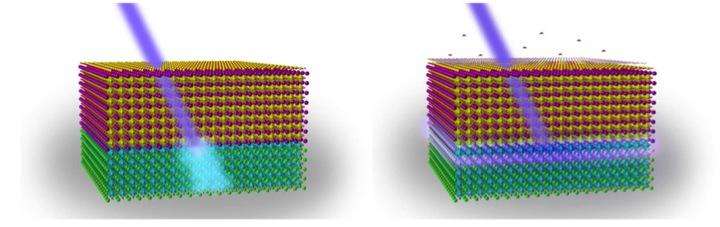
A bit of stray moisture during an experiment tipped off scientists about the strange behavior of a complex oxide material they were studying—shedding light on its potential for improving chemical sensors, computing and information storage. In the presence of a water molecule on its surface, the layered material emits ultraviolet light from its interior. A team of researchers from Drexel University, the University of Pennsylvania, the University of California at Berkeley, and Temple University recently published its discovery that it is possible to control UV light production via a chemical reaction that functions like flipping a light switch.
While studying a sample of lanthanum aluminate film on a strontinum titanate crystal, the team, led by Drexel College of Engineering Professor Jonathan E. Spanier, Andrew M. Rappe, from Penn; Lane W. Martin, from Berkeley and Temple's Xiaoxing Xi, discovered that the sample was beginning to emit intense levels of UV light. Carefully reproducing the experimental conditions helped them realize that water molecules might be playing a role in the UV light being emitted from inside the material.
"In landmark discoveries, this interface between two electrical insulators has been shown to have an electrically conducting state, one that can be altered by water on the surface of lanthanum aluminate, and also exhibits superconducting and ferromagnetic ordering," Spanier said. "But this discovery is quite remarkable because we uncovered a chemical reaction at the surface that prompts the emission of light from the interface within—and we are able to turn it off and on again. Amazingly, we can also make it stronger by increasing the distance between the molecules and surface and the buried interface, by using thicker films for example."
Team members from Drexel, Berkeley and Temple turned to their theory collaborators on the team, led by Penn's Rappe and fellow theory researchers Fenggong Wang and Diomedes Saldana-Grego, to help interpret the results.
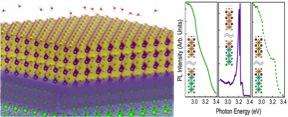
"Dissociation of water fragments on the oxide surface releases electrons that move to the buried interface, cancelling out the ionic charges," Wang said. "This puts all the light emission at the same energy, giving the observed sharp photoluminescence."
According to Rappe, this is the first report of the introduction of molecules to the surface controlling the emission of light—of any color—from a buried solid-surface interface.
"The mechanism of a molecule landing and reacting, called dissociative chemisorption, as a way of controlling the onset and suppression of light is unlike any other previously reported," Saldana-Grego said.
The team recently published its findings, in the American Chemical Society journal Nano Letters. The paper, entitled "Surface Chemically Switchable Ultraviolet Luminescence from Interfacial Two-Dimensional Electron Gas," describes their method for generating and controlling reversible ultraviolet luminescence from a two-dimensional electron gas-based semiconductor interface. This is a process they studied at length through physical testing of materials produced by collaborators at Cal and Temple, and via computer simulations by the Rappe and Spanier groups.
"We suspect that the material could be used for simple devices like transistors and sensors. By strategically placing molecules on the surface, the UV light could be used to relay information—much the way computer memory uses a magnetic field to write and rewrite itself, but with the significant advantage of doing it without an electric current," said Mohammad Islam, an assistant professor from the State University of New York at Oswego, who was on Spanier's team when he was at Drexel. "The strength of the UV field also varies with the proximity of the water molecule, this suggests that the material could also be useful for detecting the presence of chemical agents."
According to Spanier, considerably more fundamental research must be done, but this discovery can help researchers understand how electrons interact at these interfaces, and the limits of how they can use surface molecules to control the light emission.
Journal information: Nano Letters
Provided by Drexel University




















