December 17, 2015 report
New hydrogel gives clues to mechanism behind stress-stiffening-mediated mesenchymal stem cell fate
(Phys.org)—Several properties of the extracellular matrix affect cellular interaction, including stem cell differentiation. Some of these are physical properties, such as topography and matrix stiffness. In an effort to investigate these physical properties, Rajat K. Das, Veronika Gocheva, Roel Hammink, Omar F. Zouani, and Alan E. Rowan from Radbound University in The Netherlands, and the company Histide in Switzerland designed a model 3D hydrogel polymer system that demonstrates how stress-stiffening in the extracellular matrix causes human mesenchymal stem cells to favor osteogenesis over adipogenesis. Using their new model system, they determined that the expression of DCAMKL1, a microtubule associated protein, is decreasing when stress-stiffness is higher, demonstrating a novel pathway in which microtubule dynamics affects cell fate. Their work appears in Nature Materials.
The extracellular matrix (ECM) plays an important role in controlling what happens within a cell by way of physical properties like stiffness and topography. This type of interaction in which mechanical properties lead to a series of biochemical activity is known as mechanotransduction. One are of interest is how ECM stress-stiffness influences stem cell differentiation.
In order to study the mechanism behind stiffness-induced cell differentiation, researchers have tried to mimic the ECM environment. Prior studies employed several different systems to do this, including ECM-protein-derived hydrogels, synthetic polymers, and elastomeric mircopost arrays. Each of these has its strengths and weakness in how well it replicates the ECM.
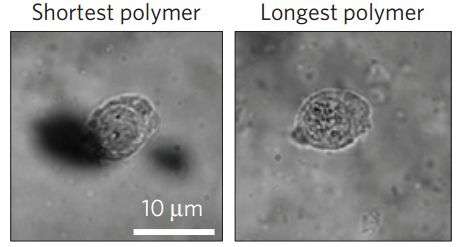
Das, et al. have designed a new type of cell culture system to mimic a three-dimensional ECM. They developed a thermoresponsive hydrogel from oligo(ethylene)glycol polyisocyanopeptides (PICs) that they can use to study the effects of stress-stiffening on human mesenchymal stem cells (hMSCs) in a three-dimensional setting by changing the length of the ethylene glycol portion of the polymer.
After making their synthetic polymers, they were functionalized with known stem cell adhesives. Analysis of the polymers showed that all of the polymers were soft and exhibited similar stiffness with critical stress measurements falling within a biologically relevant range. Additionally, critical stress was found to increase linearly as a function of polymer chain length.
Das, et al. then used their hydrogel polymers to investigate how stress-stiffening affected hMSC differentiation. Cells were cultured in a 3D matrix of the hydrogel surrounding the hMSCs. Markers for differentiation appeared after 96 hours. The cultures were tested for markers for osteogenesis and adipogenesis. Das, et al. found that cells cultured in the gel with the lowest critical stress demonstrated adipogenesis, while cells cultured in higher critical stress progressively favored osteogenesis over adipogenesis. Additional studies using RGD-modified polymers in the presence of antibodies showed that ligand interactions played an important role in mediating how stress-stiffening informs differentiation.
Das, et al. suspected that microtubule dynamics likely plays a role in stem cell fate based on the results of treating their cells with cytochalasin D, actin polymerization inhibitor, and Taxol, a microtubule stabilizing agent. They decided to investigate how exactly microtubule dynamics is at work in this system by looking at the role of DCAMKL1 and RUNX2 in stress-stiffening and hMSC cell fate. DCAMKL1 is a known microtubule-associated protein and represses RUNX2. RUNX2 is an early osteogenesis marker.
They found that DCAMKL1 expression was lowest in the longest polymers, those with highest critical stress, and its expression increased in gels with lower critical stress. Additionally, RUNX2 expression was not observed in low critical stress polymers, but was in the higher ones. Analysis confirmed a switch-like relationship between DCAMKL1 and RUNX2 and correlates to hMSCs' preference for osteogenesis in the presence of higher stress-stiffening. The authors report that this is the first time DCAMKL1, a microtubule-associated protein, has been shown to be involved in stress-stiffening-mediated mechanotransduction pathway and demonstrates how microtubule dynamics is involved in hMSC cell fate.
This study provides a new three-dimensional hydrogel for investigating stress-stiffening, and using this new hydrogel, Das, et al. were able to identify a new mechanotransduction pathway that controls hMSC differentiation that is distinctly different from two-dimensional systems.
More information: Rajat K. Das et al. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels, Nature Materials (2015). DOI: 10.1038/nmat4483
Abstract
Bulk matrix stiffness has emerged as a key mechanical cue in stem cell differentiation. Here, we show that the commitment and differentiation of human mesenchymal stem cells encapsulated in physiologically soft (~0.2–0.4 kPa), fully synthetic polyisocyanopeptide-based three-dimensional (3D) matrices that mimic the stiffness of adult stem cell niches and show biopolymer-like stress stiffening, can be readily switched from adipogenesis to osteogenesis by changing only the onset of stress stiffening. This mechanical behaviour can be tuned by simply altering the material's polymer length whilst maintaining stiffness and ligand density. Our findings introduce stress stiffening as an important parameter that governs stem cell fate in a 3D microenvironment, and reveal a correlation between the onset of stiffening and the expression of the microtubule-associated protein DCAMKL1, thus implicating DCAMKL1 in a stress-stiffening-mediated, mechanotransduction pathway that involves microtubule dynamics in stem cell osteogenesis.
Journal information: Nature Materials
© 2015 Phys.org