December 28, 2015 report
Carbon doped with nitrogen dramatically improves storage capacity of supercapacitors
(Phys.org)—A team of researchers working in China has found a way to dramatically improve the energy storage capacity of supercapacitors—by doping carbon tubes with nitrogen. In their paper published in the journal Science, the team describes their process and how well the newly developed supercapacitors worked, and their goal of one day helping supercapacitors compete with batteries.
Like a battery, a capacitor is able to hold a charge, unlike a battery, however, it is able to be charged and discharged very quickly—the down side to capacitors is that they cannot hold nearly as much charge per kilogram as batteries. The work by the team in China is a step towards increasing the amount of charge that can be held by supercapacitors (capacitors that have much higher capacitance than standard capacitors—they generally employ carbon-based electrodes)—in this case, they report a threefold increase using their new method—noting also that that their supercapacitor was capable of storing 41 watt-hours per kilogram and could deliver 26 kilowatts per kilogram to a device.
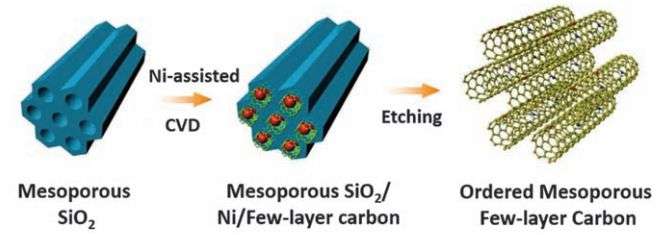
The new supercapacitor was made by first forming a template made of tubes of silica. The team then covered the inside of the tubes with carbon using chemical vapor deposition and then etched away the silica, leaving just the carbon tubes, each approximately 4 to 6 nanometers in length. Then, the carbon tubes were doped with nitrogen atoms. Electrodes were made from the resulting material by pressing it in powder form into a graphene foam. The researchers report that the doping aided in chemical reactions within the supercapacitor without causing any changes to its electrical conductivity, which meant that it was still able to charge and discharge as quickly as conventional supercapcitors. The only difference was the dramatically increased storage capacity.
Because of the huge increase in storage capacity, the team believes they are on the path to building a supercapacitor able to compete directly with batteries, perhaps even lithium-ion batteries. They note that would mean being able to charge a phone in mere seconds. But before that can happen, the team is looking to industrialize their current new supercapacitor, to allow for its use in actual devices.
More information: T. Lin et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage, Science (2015). DOI: 10.1126/science.aab3798
ABSTRACT
Carbon-based supercapacitors can provide high electrical power, but they do not have sufficient energy density to directly compete with batteries. We found that a nitrogen-doped ordered mesoporous few-layer carbon has a capacitance of 855 farads per gram in aqueous electrolytes and can be bipolarly charged or discharged at a fast, carbon-like speed. The improvement mostly stems from robust redox reactions at nitrogen-associated defects that transform inert graphene-like layered carbon into an electrochemically active substance without affecting its electric conductivity. These bipolar aqueous-electrolyte electrochemical cells offer power densities and lifetimes similar to those of carbon-based supercapacitors and can store a specific energy of 41 watt-hours per kilogram (19.5 watt-hours per liter).
Journal information: Science
© 2015 Phys.org