Antimicrobials generated using a multidisciplinary fragment-based strategy destroy drug-resistant bacterial membranes
A weapon in the battle against antibiotic resistance has been developed by A*STAR and SERI researchers, who have come up with a strategy for rational design of antimicrobials against multidrug-resistant pathogens, such as the gram-positive methicillin-resistant Staphylococcus aureus (MRSA).
Antibiotic resistance is a worldwide growing problem. Traditional antibiotics target specific intracellular microbial proteins, which are easily mutated. These mutations then alter recognition sites, which prevent drug molecules from killing bacteria or controlling their growth.
Membrane-active antimicrobials are expected to thwart this resistance by selectively penetrating and disrupting bacterial membranes, which are more difficult to reconfigure than proteins. However, the mechanism for this disruption remains unclear. A lack of general design principles has limited the development of membrane-active antimicrobials as a viable tool.
Now, teams led by Chandra Verma from the A*STAR Bioinformatics Institute and collaborator Roger Beuerman from the Singapore Eye Research Institute have developed a combined computational and experimental strategy for the rational design and synthesis of these next-generation antimicrobials.
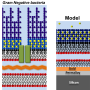
The researchers focused on the bacterial inner membrane to generate anti MRSA drug prototypes. By dividing the membrane into fragments according to wettability, they identified one hydrophobic—or water-repelling—section, sandwiched between two negatively charged regions. Next, they constructed a model comprising a hydrophobic core bearing positively charged terminal groups, which interact with the fragments. Finally, they derived the prototypes from this model using the natural substance xanthone as the core.
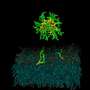
The prototypes caused the bacterial membranes to leak, which demonstrated their antimicrobial activity. The higher leakage and permeation they displayed in the presence of bacterial membranes compared with their mammalian analogs was consistent with a low toxicity toward mammalian membranes.
The researchers discovered that the antimicrobial action mechanism followed an adsorption–translocation–disruption sequence. Lead author, Jianguo Li, explains that the drug molecules initially took on a U-shaped configuration, promoting both electrostatic interactions between their terminal groups and outer membrane layer, or 'leaflet', and hydrophobic core insertion. Their accumulation gradually neutralized the outer leaflet, inducing membrane deformation and electrostatic attraction from the inner leaflet. This caused one of the antimicrobial terminal groups to cross the hydrophobic membrane section to interact with the inner leaflet, changing drug configuration and altering both membrane interfaces.
"A certain number of membrane-active antimicrobials currently in clinical trials, such as XF-73, LTX-109, and brilacidin, match our model," says Li. "This is quite exciting because it is the first reported instance of membrane-based fragment assembly," he adds.
The team is currently working on the development of antimicrobials that simultaneously target gram-positive and gram-negative pathogens. "We hope that the same concept can be extended to cancer cell membranes," says Verma.
More information: Jianguo Li et al. A novel fragment based strategy for membrane active antimicrobials against MRSA, Biochimica et Biophysica Acta (BBA) - Biomembranes (2015). DOI: 10.1016/j.bbamem.2015.01.001