November 6, 2015 report
Structure and configuration of Jurassic pigments reveal unique naturally-occurring boron metabolites
(Phys.org)—A group of researchers from the University of Göttingen, the Max Planck Institute for Biophysical Chemistry, and the University of Linz has determined the structure and origin of Jurassic period borolithochromes. It is the first reported boronated aromatic polyketide, whose structural similarity to modern-day clostrubin A, a secondary metabolite, suggests that borolithochromes may come from ancient bacteria. Their report appears in the Journal of the American Chemical Society.
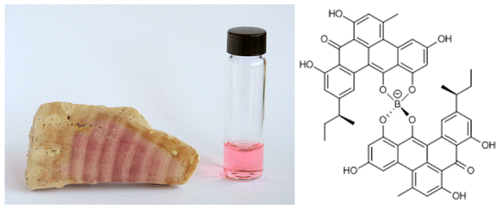
The pink Jurassic period alga, Solenpora jurassica, gets its color from a boron-containing organic compound whose structure has proved elusive for researchers to determine because there is so little preserved organic material available. Sensitive techniques, such as mass spectrometry, provide information about the boron compound's composition, but it is not enough to determine its structure and configuration. NMR would provide the needed information to determine the compound's structure but its limit of detection is not typically low enough for such small samples.
Wolkenstein, et al. used a technique called micro cryoprobe NMR. Micro cryoprobe NMR is more sensitive than room temperature NMR with limits of detection in the nanogram range. They isolated the boron compounds from a Solenpora sample via using high pressure liquid chromatography. For each of their isolated boron compounds, mass spectrometry indicated a molecular formula with about twice as many hydrogens as indicated in micro cryoprobe NMR. This suggested a symmetry structure in which two ligands surround the boron center.
They conducted additional NMR studies as well as simulation studies of the various isolated boron compounds. All of them seemed to be substituted variations on the basic forty-eight carbon compound. Their data allowed for the elucidation of the conjugated pentacyclic ligand as well as identification of substituents. They were able to isolate one compound with molecular formula C52H40O12B in sufficient yield to determine its structure absolutely. This structure had a sec-butyl group and a chiral axis that was studied using circular dichroism. They found that for either diastereomer, the chiral center of the sec-butyl group, is always left-handed (S), which indicates that this compound is of biological origin.
Additional structural features indicated that the borolithochrome are polyketides, which would make them the first boronated aromatic polyketides. The borolithochromes are unique spiroborates with two exceptional pentacyclic aromatic ligands. Not only is a naturally occurring boron-containing compound a rare find, but the ligand structure was unknown until the recently discovery of the structure of antibiotic polyketide called clostrubin A.
The structural similarity between the Jurassic borolithochrome and clostrubin A has important implications for understanding Jurassic period organisms. Wolkenstein, et al. a proposed possible pathway for the biosynthesis of the Jurassic borolithochromes based on the biosynthetic pathway of clostrubin A and other known polyketide reactions. Additionally, since clostrubin A is an antibiotic polyketide produced by a bacterium, the borolithochromes likely originated from ancient bacteria.
More information: Klaus Wolkenstein et al. Structure and Absolute Configuration of Jurassic Polyketide-Derived Spiroborate Pigments Obtained from Microgram Quantities, Journal of the American Chemical Society (2015). DOI: 10.1021/jacs.5b08191
Abstract
Complete structural elucidation of natural products is often challenging due to structural complexity and limited availability. This is true for present-day secondary metabolites, but even more for exceptionally preserved secondary metabolites of ancient organisms that potentially provide insights into the evolutionary history of natural products. Here, we report the full structure and absolute configuration of the borolithochromes, enigmatic boron-containing pigments from a Jurassic putative red alga, from samples of less than 50 μg using microcryoprobe NMR, circular dichroism spectroscopy, and density functional theory calculations and reveal their polyketide origin. The pigments are identified as spiroborates with two pentacyclic sec-butyl-trihydroxy-methyl-benzo[gh]tetraphen-one ligands and less-substituted derivatives. The configuration of the sec-butyl group is found to be (S). Because the exceptional benzo[gh]tetraphene scaffold is otherwise only observed in the recently discovered polyketide clostrubin from a present-day Clostridium bacterium, the Jurassic borolithochromes now can be unambiguously linked to the modern polyketide, providing evidence that the fossil pigments are almost originally preserved secondary metabolites and suggesting that the pigments in fact may have been produced by an ancient bacterium. The borolithochromes differ fundamentally from previously described boronated polyketides and represent the first boronated aromatic polyketides found so far. Our results demonstrate the potential of microcryoprobe NMR in the analysis of previously little-explored secondary metabolites from ancient organisms and reveal the evolutionary significance of clostrubin-type polyketides.
Journal information: Journal of the American Chemical Society
© 2015 Phys.org