Scandium trifluoride shrinks when warm, lends understanding of volume-changing materials

Most materials swell when they warm, and shrink when they cool. But UConn physicist Jason Hancock has been investigating a substance that responds in reverse: it shrinks when it warms.
Although thermal expansion, and the cracking and warping that often result, are an everyday occurrence – in buildings, bridges, electronics, and almost anything else exposed to wide temperature swings – physicists have trouble explaining why solids behave that way.
Research by Hancock and his colleagues into scandium trifluoride, a material that has negative thermal expansion, scheduled for publication in Physical Review B, may lead to a better understanding of why materials change volume with temperature at all, with potential applications such as more durable electronics.
The classical way to think about solids like glass, metal, and rock imagines them made of atoms hooked together by springs. The springs stretch and flex in response to heat. But because each spring, when it expands, puts pressure on its neighboring springs—and all those neighboring springs expand the same amount and exert the same pressure on the first spring and all their own neighboring springs—the forces they exert on each other should be symmetrical, and the material should neither expand nor contract.
"In many ways, the model is good," says Hancock. "It explains inelastic scattering of neutrons and x-rays, lots of other optical effects, the speed of sound waves, aspects of elasticity and heat conduction, even the transition temperature of some superconductors."
But it doesn't do a good job of explaining thermal expansion.
Hancock and graduate student Sahan Handunkanda decided to look at scandium trifluoride because its odd behavior might give them some clues on what to look for in more typical materials. Not only does scandium trifluoride drastically shrink as it warms over a huge range of temperature (almost 1,100K or 2,000 F); it also keeps the same, stable cubic crystal structure over an even larger temperature range, from near absolute zero to 1,800 degrees Kelvin (2,780 degrees Fahrenheit), at which point it melts. Very few materials can boast of being so stable; most have some kind of phase change, during which their atoms shift positions, at least once when they're warmed over 2,780 degrees Fahrenheit.
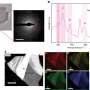
To figure out what was happening inside scandium trifluoride, the researchers decided to use x-rays to show how the atoms in the crystal moved at very low temperatures, close to absolute zero. To do this right, they needed a perfect crystal of scandium trifluoride, something that was very difficult to get. After searching, they found possibly the only source for the perfect crystals they needed: a group at Kirensky Institute of Physics in Krasnoyarsk, Siberia, led by Vladimir Voronov. Voronov agreed to send them crystals by mail, and they scheduled x-ray beam time at the Advanced Photon Source at Argonne National Laboratory.
The researchers shone a beam of x-rays onto the perfect crystal. They knew exactly how much energy the x-rays had going in to the crystal, and they carefully tracked how much energy the x-rays had coming out. By tracking the amount of energy the x-rays lost, the angle they entered the crystal, and the angle they emerged, the researchers could calculate how the scandium trifluoride atoms moved.
"When x-rays bounce from the sample, they make little splashes of vibration in the lattice," Hancock says. Columns of scandium trifluoride molecules, each shaped like a little octahedron, seemed to be rotating in place, even at close to zero degrees.
The ease with which the columns twisted at close to zero temperature is unusual—the structure is 'softer' than most materials at zero Kelvin. The observed softness of scandium fluoride's molecular structure suggests it's about to undergo a phase shift, but it never quite gets there, even near absolute zero. Such a shift near absolute zero is called a quantum phase transition, and is an area of intense research activity in physics, mainly because such transitions often challenge current theoretical understanding of how materials work.
The clues uncovered in this study suggest that there could be a deep relationship between quantum forces and the giant shrinkage the material experiences as it warms. Hancock and Handunkanda would like to explore the implications of that both experimentally and theoretically.
On a more immediate level, scandium trifluoride is a material with a future. Its crystal structure is similar to many materials used in electronics, and it's transparent, making it an interesting potential component of devices that don't shrink, crack, or break under thermal stress.
More information: "Large isotropic negative thermal expansion above a structural quantum phase transition." Phys. Rev. B 92, 134101 – Published 1 October 2015. dx.doi.org/10.1103/PhysRevB.92.134101
Journal information: Physical Review B
Provided by University of Connecticut