Light shed on the underside of the 'cocktail effect' of endocrine disruptors
Chemical substances that are safe for humans when taken in isolation can become harmful when they are combined. Three research teams bringing together researchers from Inserm and CNRS in Montpellier have elucidated in vitro a molecular mechanism that could contribute to the phenomenon known as the "cocktail effect." This study is published in the journal Nature Communications.
Every day we are exposed to many exogenous compounds such as environmental pollutants, drugs or substances in our diet. Some of these molecules, known as endocrine disruptors, are strongly suspected of interacting inappropriately with regulatory proteins in our cells, and inducing numerous physiological or metabolic disorders (cancers, obesity, diabetes, etc.). Moreover, the combination of these molecules in complex mixtures with which we are in routine contact might exacerbate their toxicity.
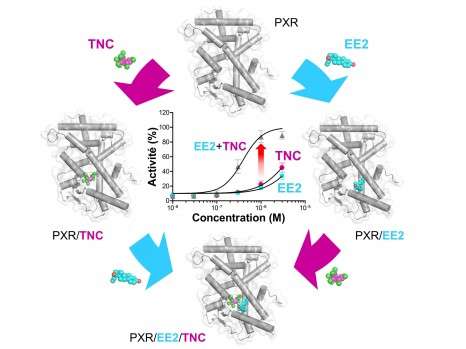
In an article to be published in Nature Communications, researchers have unveiled a mechanism that might contribute to this effect of mixing, for which no rational explanation has been offered until now. They show that some oestrogens such as ethinyloestradiol (one of the active ingredients of contraceptive pills) and organochlorine pesticides such as trans-nonachlor, although very weakly active on their own, have the ability to bind simultaneously to a receptor located in the cell nucleus, and to activate it synergistically.
Analyses at molecular level indicate that the two compounds bind cooperatively to the receptor, i.e. binding of the first molecule promotes binding of the second. This cooperativity is due to strong interactions at the level of the receptor binding site, so that the binary mixture induces a toxic effect at substantially lower concentrations than the individual molecules.
These results obtained in vitro constitute a proof of concept that opens the way to a wide field of study. There are actually about 150,000 compounds in our environment that could have unexpected effects on human health through combined action, given their recognised or assumed safety as isolated substances. If these studies are confirmed in vivo, important consequences are expected in the areas of endocrine disruption, toxicology, and the assessment of risks associated with the use of chemicals.
More information: Nature Communications, dx.doi.org/10.1038/ncomms9089
Journal information: Nature Communications