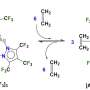
Organometal trihalide perovskite solar cells with conversion efficiencies of 20.1%
25 April 1954 was a seminal day in the history of research on solar cells. On this date scientists at Bell Labs, Murray Hill, USA, announced the invention of the world's first practical panel of solar cells for converting sunlight into electricity. The solar cells were made of silicon and had an efficiency of 6 percent—a tremendous achievement even by today's standards. Notably, the solar cells were used to power a radio transmitter and energize a motor to drive a Ferris wheel—both practical applications of solar cells.
Fast forward to 2015 and silicon-based solar panels are ubiquitous. They have become an indispensable means of generating energy for human habitats, space exploration, and wearable devices such as wrist watches.
Now, at the research level, scientists are innovating to produce even more efficient solar cells at low cost, using environmentally friendly materials. Notably, recent reports of solar cell conversion efficiencies of 20.1% for cells fabricated using organometal trihalide perovskite have caught the imagination of scientists in the search for high performance, cheap solar cells.
"This very high 20% efficiency exhibited by organolead halide perovskite solar cells has galvanized scientists to focus on understanding the physics of this material system," says Qing Shen, associate professor at the Department of Engineering Science, Faculty of Informatics and Engineering at UEC. "Two of the main issues to resolve are clarifying why organolead halide perovskites yield such a high efficiency, and potential alternatives to lead based perovskites."
The material properties governing the high conversion efficiency of perovskite-based organic/inorganic solid state solar cells (OIHSCs ) include their direct band gap and high optical absorption; large dielectric coefficient leading to smaller exciton binding energy; long photoexcited carrier lifetimes; no deep level defects; and very small Urbach energy.
"Our research is focused on clarifying the physical properties of this material that are responsible for such a high conversion efficiency," says Shen. "More specially, we are studying the relationship between carrier lifetime, charge separation and charge recombination dynamics and the performance of photovoltaic perovskite-based OIHSCs."
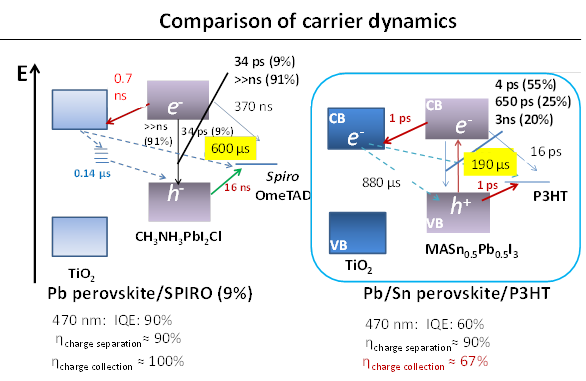
Shen has developed a dedicated transient absorption (TA) system to measure charge separation and recombination dynamics from the sub-picoseond to milli-second range. Recent experiments shows perovskite CH3NH3PbIxCl3-x to show long carrier lifetimes in the micro-second range and charge separation efficiency of TiO2/CH3NH3PbIxCl3-x/Spiro to be as high as 90%.
"Decreasing the recombination at TiO2/Spiro OMeTAD interface is a critical factor for improving the efficiency," says Shen. "Other issues are devising methods to suppress recombination, surface passivation of TiO2, and interface engineering."
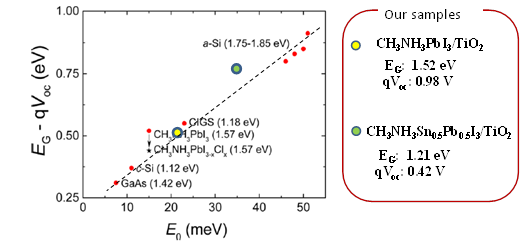
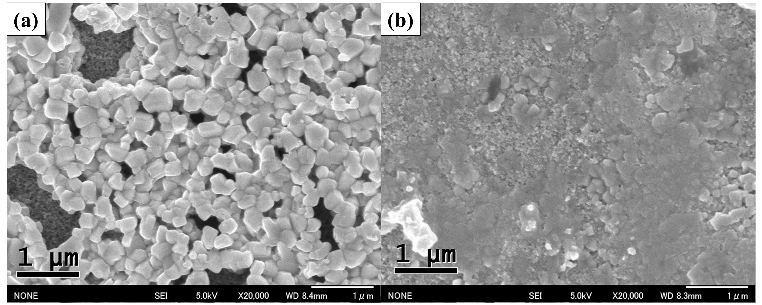
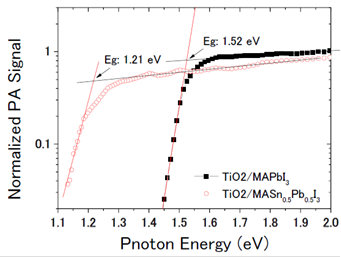
Further improvements in the efficiency of these solar cells are expected by capturing more light from the near infra-red region of the spectrum. Professor Shuzi Hayase of the Kyushu Institute of Technology, leader of the JST CREST research group—which Shen has recently joined— has reported that this can be achieved by using a 'Sn/Pb cocktail perovskite' in the form of CH3NH3SnxPbI1-xI3 perovskite solar cells that cover the spectral range up to 1060 nm.
Shen and colleagues have combined transient absorption with photoacoustic spectroscopy to analyze the properties of these materials to improve the Jsc, Voc and FF of Sn/Pb cocktail perovskite solar cells. Initial findings indicate that better solar cells could be produced by reducing non-radiative recombination by improving the crystalline quality of the Pb/Sn perovskites and suitable passivation of the TiO2/Sn/Pb perovskite interface, and realization of pinhole-free perovskite films.
"This is an exciting time to be involved in solar cell research," says Shen. "This is interdisciplinary research requiring many different skills. I am currently working with Professor Shuzi Hayase at Kyushu Institute of Technology and other collaborators on producing high efficiency, inexpensive perovskite organic/inorganic solar cells."
More information: "Optical Absorption, Charge Separation and Recombination Dynamics in Sn/Pb Cocktail Perovskite Solar Cells and Their Relationships to Photovoltaic Performances," J. Mater. Chem. A, 2015, 3, 9308-9316, DOI: 10.1039/C5TA01246E
"Charge transfer and recombination at the metal oxide/CH3NH3PbClI2/spiro-OMeTAD interfaces: uncovering the detailed mechanism behind high efficiency solar cells," Phys. Chem. Chem. Phys., 2014,16, 19984-19992, DOI: 10.1039/C4CP03073G
"All-Solid Perovskite Solar Cells with HOCO-R-NH3+I- Anchor-Group Inserted between Porous Titania and Perovskite," J. Phys. Chem. C, 2014, 118 (30), pp 16651-16659, DOI: 10.1021/jp412627n
"Control of Charge Dynamics through a Charge-SeparationInterface for All-Solid Perovskite-Sensitized Solar Cells," CHEMPHYSCHEM, 2014. DOI: 10.1002/cphc.201301153
"CH3NH3SnxPb(1−x)I3 Perovskite Solar Cells Covering up to 1060 nm," J. Phys. Chem. Lett.. Vol. 5, 1004−1011 (2014) dx.doi.org/10.1021/jz5002117
Journal information: Journal of Materials Chemistry A , ChemPhysChem
Provided by UEC Tokyo