A step toward clothing that guards against chemical warfare agents
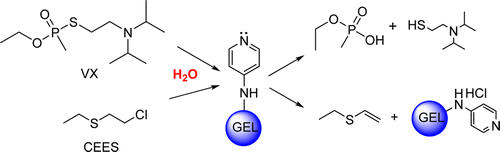
Recent reports of chemical weapons attacks in the Middle East underscore the urgent need for new ways to guard against their toxic effects. Toward that end, scientists report in the journal ACS Applied Materials & Interfaces a new hydrogel coating that neutralizes both mustard gas and nerve agent VX. It could someday be applied to materials such as clothing and paint.
Toxic chemicals have been used as weapons since ancient times, but it wasn't until World War I that they were released in large-scale attacks. Despite international efforts to ban them, chemical warfare agents (CWA) are still deployed. Scientists have developed some substances that can neutralize CWAs, but they lose their effectiveness when incorporated into practical coatings such as paint. Lev Bromberg, a research scientist in T. Alan Hatton's group, and other colleagues wanted to come up with a better solution.
The researchers developed hydrogel materials that completely broke down the nerve gas VX—one of the most dangerous and persistent CWAs—in less than 20 minutes. The materials also quickly degraded mustard gas and soman, a nerve agent that was reportedly used in the 1980s during the Iran-Iraq war. And, the researchers say, the hydrogels could be applied to fabrics or other materials without losing their ability to neutralize CWAs.
More information: Nucleophilic Polymers and Gels in Hydrolytic Degradation of Chemical Warfare Agents, ACS Appl. Mater. Interfaces, Article ASAP. DOI: 10.1021/acsami.5b06905
Abstract
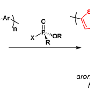
Water- and solvent-soluble polymeric materials based on polyalkylamines modified with nucleophilic groups are introduced as catalysts of chemical warfare agent (CWA) hydrolysis. A comparative study conducted at constant pH and based on the criteria of the synthetic route simplicity, aqueous solubility, and rate of hydrolysis of CWA mimic, diisopropylfluorophosphate (DFP), indicated that 4-aminopyridine-substituted polyallylamine (PAAm-APy) and polyvinylamine substituted with 4-aminopyridine (PVAm-APy) were advantageous over 4-pyridinealdoxime-modified PVAm and PAAm, poly(butadiene-co-pyrrolidinopyridine), and PAAm modified with bipyridine and its complex with Cu(II). The synthesis of PVAm-APy and PAAm-APy involved generation of a betaine derivative of acrylamide and its covalent attachment onto the polyalkylamine chain followed by basic hydrolysis. Hydrogel particles of PAAm-APy and PVAm-APy cross-linked by epichlorohydrin exhibited pH-dependent swelling and ionization patterns that affected the rate constants of DFP nucleophilic hydrolysis. Deprotonation of the aminopyridine and amine groups increased the rates of the nucleophilic hydrolysis. The second-order rate of nucleophilic hydrolysis was 5.5- to 10-fold higher with the nucleophile-modified gels compared to those obtained by cross-linking of unmodified PAAm, throughout the pH range. Testing of VX and soman (GD) was conducted in 2.5–3.7 wt % PVAm-APy suspensions or gels swollen in water or DMSO/water mixtures. The half-lives of GD in aqueous PVAm-APy were 12 and 770 min at pH 8.5 and 5, respectively. Addition of VX into 3.5–3.7 wt % suspensions of PVAm-APy in DMSO-d6 and D2O at initial VX concentration of 0.2 vol % resulted in 100% VX degradation in less than 20 min. The unmodified PVAm and PAAm were 2 orders of magnitude less active than PVAm-APy and PAAm-APy, with VX half-lives in the range of 24 h. Furthermore, the PVAm-APy and PAAm-APy gels facilitated the dehydrochlorination reaction of sulfur mustard (HD) and its analogue 2-chloroethyl ethylsulfide (CEES). The ability of the reported aminopyridine-modified polyalkylamine materials to degrade the most persistent of CWAs, coupled with aqueous solubility, and the presence of numerous amino groups that provide convenient "handles" for covalent attachment on polymeric and inorganic supports yields promise for applications such as protective fabric and textile treatment and components of decontaminating materials.
Journal information: ACS Applied Materials and Interfaces
Provided by American Chemical Society