August 24, 2015 report
Solid-state molecular switches using redox active molecules in a porous crystal
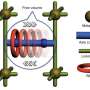
(Phys.org)—A group of researchers have provided a proof-of-concept procedure for making a solid-state molecular-sized switch. They combined a mechanically interlocked molecule with a pre-synthetized metal-organic framework (MOF).
Mechanically interlocked molecules have several features that make them ideal candidates for molecular switches. These interlocked molecules, known as rotaxanes or catenanes, typically involve two molecular components that have distinct orientations based on interactions between them. Scientists can control which orientation the interlocked molecules take using stimuli, such as electrochemical potential or light. Redox active interlocked molecules are compelling candidates for a molecular switch. However, in solution, these molecules are unpredictable, and when it comes to designing circuitry, controlling the molecular switch is vital.
This is where MOFs can be helpful. MOFs are highly porous materials. Other molecules can be trapped within the pores, and these pores can be chemically tailored to select for certain molecules. A group of researchers from Northwestern University in collaboration with Intel Labs and King Abdulaziz University have successfully captured a redox-active rotaxane within the pores of a premade MOF via post-synthetic building block replacement. Their paper is published in the Proceedings of the National Academy of Sciences.
"As chemists, we have become proficient at manipulating molecular switches and rudimentary molecular machines in solution during the past quarter of a century," said co-author Dr. Fraser Stoddart, "What we would really like to be able to do now is mount these switches inside porous solids in a rapid and changeable manner. This task has turned out to be easier said than done!"
Previous research with mechanically interlocked molecules that are stabilized in a metal-organic framework involved building the MOF around the interlocked molecules in such a way that the "struts" of the MOF were also part of the interlocked molecule. There were several drawbacks to this process, including the need to optimize conditions so that a crystal would form, and in some cases, the molecules did not move, a necessary property for molecular switching.
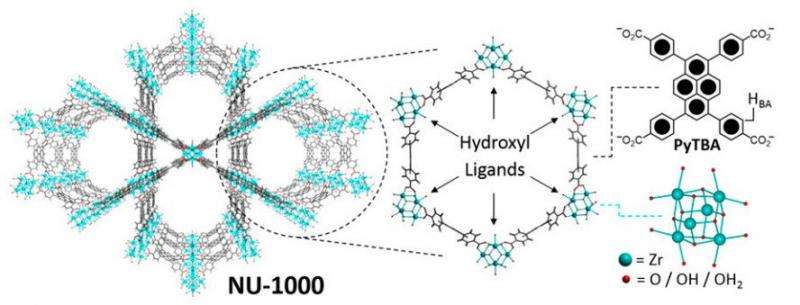
McGonigal, et al. wanted to design a mechanically interlocked molecule that would fit within the pores of a pre-made MOF, rather than incorporate the molecule into the architecture of a novel MOF. They accomplished this by capturing a redox-active rotaxane within the pores of a zirconium-based MOF (NU-1000) post-synthetically using solvent-assisted ligand incorporation (SALI). Their model system provides a proof-of-concept.
The first step in their procedure was to design the system. NU-1000 has hydroxyl groups that are oriented toward the center of a hexagonal channel. McGonigal, et al. realized that these hydroxyl groups could be displaced by carboxyl groups present in a rotaxane using solvent-assisted ligand incorporation (SALI). In this way, the carboxyl groups act as attachment points for the rotaxane.
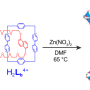
Their rotaxane needed to serve as a model system and work with their SALI procedure. They used a viologen-based molecule with a "stopper" on one end to ensure it becomes mechanically interlocked. Viologens are linear molecules comprised of two aromatic rings (pyridinium). This viologen was substituted with benzoic acid on one end to coordinate to the zirconium, and a benzene ring substituted with two tert-butyl groups to act as "stoppers." Under reducing conditions, the viologen will complex with a tetracationic macrocycle containing two viologens as a result of radical-radical pairing interactions. The resulting semirotaxane was verified using spectroscopic methods.
The newly formed mechanically interlocked molecule was then coaxed into the functionalized MOF. The semirotaxane diffuses through the channel of the MOF and is captured by coordinating to the Zr clusters. The t-butyl bulky end served as a "stopper."
Characterization studies verified that the semirotaxane was successfully incorporated into a post-synthetic MOF. The system was more stable than the semirotaxane in solution. Further, their studies indicated that the proportion of semirotaxane-to-MOF was not as expected. Rather than four semirotaxanes to one zirconium cluster, they observed a 1:1 ratio. Modeling showed that fewer dumbbells per channel allowed for packing in the channel without distorting the MOF architecture.
Finally, electrochemical studies were done to confirm the switching behavior of the semirotaxane. McGonigal, et al. used electrophoretic deposition to deposit microcrystals of NU-1000 on conductive fluorine-doped tin oxide transparent electrodes. They then used their SALI procedure to obtain a thin film of the semirotaxane-MOF compound. Electrochemical studies confirmed that a large portion of the semirotaxane maintains its redox activity.
According to Dr. Stoddart it was the simplicity of this this method that his group found appealing, "We make the molecular switches in solution, where we are already very comfortable with the chemistry, and then just mix them with some porous crystals. The switches insert themselves into the porous interiors of the crystals by means of some strong chemical bonds and that's it! Switchable molecules slotted into crystals full of apace at the drop of a hat!"
More information: "Electrochemically addressable trisradical rotaxanes organized within a metal-organic framework" PNAS DOI: 10.1073/pnas.1514485112
Abstract
The organization of trisradical rotaxanes within the channels of a Zr6-based metal–organic framework (NU-1000) has been achieved postsynthetically by solvent-assisted ligand incorporation. Robust ZrIV–carboxylate bonds are forged between the Zr clusters of NU-1000 and carboxylic acid groups of rotaxane precursors (semirotaxanes) as part of this building block replacement strategy. Ultraviolet–visible–near-infrared (UV-Vis-NIR), electron paramagnetic resonance (EPR), and 1H nuclear magnetic resonance (NMR) spectroscopies all confirm the capture of redox-active rotaxanes within the mesoscale hexagonal channels of NU-1000. Cyclic voltammetry measurements performed on electroactive thin films of the resulting material indicate that redox-active viologen subunits located on the rotaxane components can be accessed electrochemically in the solid state. In contradistinction to previous methods, this strategy for the incorporation of mechanically interlocked molecules within porous materials circumvents the need for de novo synthesis of a metal–organic framework, making it a particularly convenient approach for the design and creation of solid-state molecular switches and machines. The results presented here provide proof-of-concept for the application of postsynthetic transformations in the integration of dynamic molecular machines with robust porous frameworks.
Journal information: Proceedings of the National Academy of Sciences
© 2015 Phys.org