Using computational methods, scientists tailor and adapt proteins to mine uranium from seawater
Researchers developed a protein-based, genetically encodable system that can bind water-soluble uranium with exceedingly high affinity and selectivity. This also is the first time that a protein has been designed with these characteristics using exclusively natural amino acids.
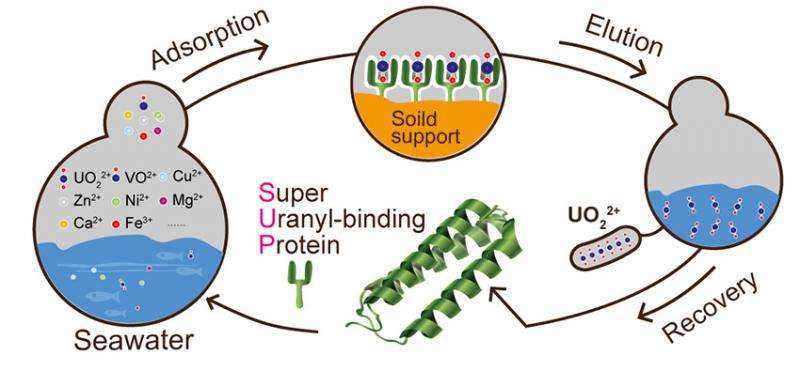
This is the first known demonstration of a bacterial system used to mine ocean-based uranium that reduces the expense while increasing the selectivity of current methods available. The overall method developed could find broad applications in sequestration and bioremediation of water-soluble uranium and similar transuranic elements. This biotechnology method could also have similar applications to other low-concentration ions in solution.
Uranium plays an important role in the search for alternative energies to fossil fuels; however, uranium resources on land are limited. The oceans are estimated to contain 1,000 times as much uranium as is buried in deposits on land, but unfortunately, the uranium in the ocean is in the form of water-soluble uranyl (UO22+) which is present at a very low concentration (~13.7 nM). The uranyl is bound by carbonate and other anions, with the added complication that seawater also contains various metal ions at high concentrations, making separating the uranium extremely complex.
After years of trying to find an efficient and affordable way to extract uranyl, researchers at the University of Chicago, Peking University, and Argonne National Laboratory turned to biology. There are no naturally occurring proteins known to bind uranyl, but by adapting a computational screening strategy for protein-protein interactions, a potential uranyl-binding motif was designed. The scientists used the motif to search the Protein Data Bank for proteins that could accommodate or be adapted to accommodate uranyl. One candidate in initial binding experiments showed promise and was further optimized. The engineered, thermally stable protein called Super Uranyl-binding Protein (SUP) binds uranyl tightly (Kd of 7.4 femtomolar) and with high selectivity (>10,000-fold selectivity over other metal ions). The SUP was also confirmed to contain the computationally designed structural features through examination of the protein crystal structure. This protein can repeatedly sequester 30 to 60% of the uranyl in synthetic sea water and thus provides a much needed advance in the isolation of uranyl from seawater.
More information: "A protein engineered to bind uranyl selectively and with femtomolar affinity." Nature Chemistry 6, 236-241 (2014). DOI: 10.1038/nchem.1856
Journal information: Nature Chemistry
Provided by US Department of Energy


















