April 15, 2015 report
Quantum Criticality in life's proteins (Update)
(Phys.org)—Stuart Kauffman, from the University of Calgary, and several of his colleagues have recently published a paper on the Arxiv server titled 'Quantum Criticality at the Origins of Life'. The idea of a quantum criticality, and more generally quantum critical states, comes perhaps not surprisingly, from solid state physics. It describes unusual electronic states that are are balanced somewhere between conduction and insulation. More specifically, under certain conditions, current flow at the critical point becomes unpredictable. When it does flow, it tends to do so in avalanches that vary by several orders of magnitude in size.
Ferroelectric metals, like iron, are one familiar example of a material that has classical critical point. Above a critical temperature of 1043 degrees K the magnetization of iron is completely lost. In the narrow range approaching this point, however, thermal fluctuations in the electron spins that underly the magnetic behavior extend over all length scales of the sample—that's the scale invariance we mentioned. In this case we have a continuous phase transition that is thermally driven, as opposed to being driven by something else like external pressure, magnetic field, or some kind of chemical influence.
Quantum criticality, on the other hand, is usually associated with stranger electronic behaviors—things like high-temperature superconductivity or so-called heavy fermion metals like CeRhIn5. One strange behavior in the case of heavy fermions, for example, is the observation of large 'effective mass'—mass up to 1000 times normal—for the conduction electrons as a consequence of their narrow electronic bands. These kinds of phenomena can only be explained in terms of the collective behavior of highly correlated electrons, as opposed to more familiar theory based on decoupled electrons.
Experimental evidence for critical points in quantum phase transitions of materials like CeRhIn5 has only recently been found. In this case the so-called "Fermi surface," a three-dimensional map representing the collective energy states of all electrons in the material, was seen to have large instantaneous shifts at the critical points. When electrons across the entire Fermi surface are strongly coupled, unusual physics like superconductivity is possible.
The potential existence of quantum critical points in proteins is a new idea that will need some experimental evidence to back it up. Kauffman and his group eloquently describe the major differences between current flow in proteins as compared to metallic conductors. They note that in metals charges 'float' due to voltage differences. Here, an electric fields accelerate electrons while scattering on impurities dissipates their energy fixing a constant average propagation velocity.
By contrast, this kind of a mechanism would appear to be uncommon in biological systems. The authors note that charges entering a critically conducting biomolecule will be under the joint influence of the quantum Hamiltonian and the excessive decoherence caused by the environment. Currently a huge focus in Quantum biology, this kind of conductance has been seen for example, for excitons in the light-harvesting systems. As might already be apparent here, the logical flow of the paper, at least to nonspecialists, quickly devolves into the more esoteric world of quantum Hamiltonians and niche concepts like 'Anderson localization.'
To try to catch a glimpse of what might be going on without becoming steeped in formalism I asked Luca Turin, who actually holds the patent for semiconductor structures using proteins as their active element, for his take on the paper. He notes that the question of how electrons get across proteins is one of the great unsolved problems in biophysics, and that the Kauffman paper points in a novel direction to possibly explain conduction. Quantum tunnelling (which is an essential process, for example, in the joint special ops of proteins of the respiratory chain) works fine over small distances. However, rates fall precipitously with distance. Traditional hole and electron transport mechanisms butt against the high bandgap and absence of obvious acceptor impurities. Yet at rest our body's fuel cell generates 100 amps of electron current.
In suggesting that biomolecules, or at least most of them, are quantum critical conductors, Kauffman and his group are claiming that their electronic properties are precisely tuned to the transition point between a metal and an insulator. An even stronger reading of this would have that there is a universal mechanism of charge transport in living matter which can exist only in highly evolved systems. To back all this up the group took a closer look at the electronic structure of a few of our standard issue proteins like myoglobin, profilin, and apolipoprotein E.
In particular, they selected NMR spectra from the Protein Data Bank and used a technique known as the extended Huckel Hamiltonion method to calculate HOMO/LUMO orbitals for the proteins. For more comments on HOMO/LUMO orbital calculations you might look at our post on Turin's experiments on electron spin changes as a potential general mechanism of anesthesia. To fully appreciate what such calculations might imply in this case, we have to toss out another fairly abstract concept, namely, Hofstadter's butterfly as seen in the picture below.
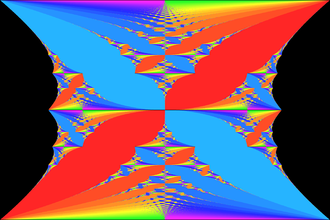
The butterfly is a graphical representation of the fractal structure of the energy spectrum for electrons in a magnetic field. The self-similarity of a fractal structure can be quantified by one of several measures of something called the fractal dimension. This is a general measure of complexity usually taken as the ratio of the change in detail to the change in scale. Originally proposed in by Hofstadler in 1976, his signature butterfly is one of the rare fractal footprints discovered in physics that has recently been experimentally observed.
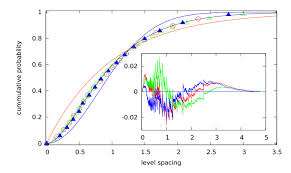
When Kauffman and his group calculated fractal correlation dimensions for their example proteins they got values precisely midway between localization (D = 0) and delocalization (D = 1) confirming that the system is critical and that the wave functions are multifractals. They also report that the same numerical value (D = 0.5) has been obtained also for 'critical quantum chaos.' Perhaps more contentious then their calculations are the suggestions that this kind of criticality (in the quantum Hamiltonian) is endemic in the evolutionary selection of biomolecules.
Their justification for this line of thought proceeds from an argument that the probability of finding such critical molecules by a quasi-random browsing of major databases and 3D crystallographic data is astronomically low. They note that the number of known small molecules and proteins is about 10^8 and the number of chemically feasible small (< 500Da) organic compounds is estimated at around 10^60. In the same vein, the number of proteins grows exponentially with the number n of amino acids as ∼ 20n (myoglobin consists of 153 amino acids) and the largest known has about n ≈ 26000. They conclude that chemical and biological evolution selected only a tiny fraction p ∼ 10−50 of possible small biomolecules and even less for proteins.
However, in asking why life just uses the molecules that does, the authors don't explicitly address the question of just how many potential small biomolecules and or proteins would be expected to quantum critical in the first place. They do note that some biomolecules are actually fairly good conductors. Some of the essential steroids which are bioactive extremely low (nanomolar) concentrations, like testosterone, fall into that category.
We might call to mind at this point that others have looked for similar kinds of extreme behaviours in other examples of life's proteins. Stuart Hameroff has been a long time champion of networks of polymerized tubulins in the conduction of information in the cells through as yet fully defined mechanisms. In particular, we should mention recent work on driving the rapidly polymerization of microtubules through external electromagnetic fields raises the question of what new kinds of physics may be at play here. I discussed some of this in more detail elsewhere the other day, together with some of the researchers here for anyone interested to read a little more.
————————
UPDATE, 4/15/2015: Guest blog comment:
Quantum criticality in living systems
by Stuart Hameroff MD,
Anesthesiology, Psychology and Center for Consciousness Studies,
The University of Arizona, Tucson, Arizona
The work by Stuart Kauffman, Gabor Vattay and colleagues makes an important contribution, showing biomolecules exist in a state of quantum criticality, poised at the edge between quantum and classical behavior. They rightfully, and correctly in my view, suggest this quantum critical feature is relevant to the fundamental nature of life. It should be noted that reductionist, functionalist and genetic approaches have yet to explain life's unitary oneness, its fundamental nature.
Quantum coherence was proposed as an intrinsic feature of life by Schrodinger in his book 'What is Life', and historically supported by Albert Szent-Gyorgyi, Herbert Frohlich and others. But artificial quantum systems are plagued by thermal and electromagnetic interactions with their environments, disrupting quantum states and causing 'decoherence'. Accordingly, laboratory quantum devices are built near absolute zero temperature, and living systems considered too 'warm, wet and noisy' for quantum coherence. However some scientists including myself and Sir Roger Penrose have thought that biology could have developed mechanisms to avoid decoherence, or perhaps that life even originated because of quantum mechanisms.
Indeed it is now recognized that proteins in plant photosynthesis utilize quantum coherence to make food. Energy from collected photons are conveyed through the plant protein by quantum electron excitations (excitons) via 8 geometrically-arrayed 'chromophores', each a non-polar group of 'pi resonance clouds'. Optimizing efficiency, the excitons propagate through all 8 chromophores simultaneously in quantum superposition.
The key to quantum coherence in photosynthesis is the non-polar, pi resonance environment in which it occurs, an environment from which, it appears, consciousness originates.
Biomolecules are generally 'amphipathic', with charged, water-soluble polar groups on one end, and an oil-like, non-polar group on the other. Oil and water don't mix. When amphipathic biomolecules self-assemble (e.g. in protein folding), the non-polar groups coalesce, forming intra-protein 'hydrophobic pockets', excluding water. The polar ends stick out into the charged, watery environment.
Non-polar hydrophobic pockets inside proteins are composed primarily of pi resonance clouds like the phenyl and indole rings of aromatic amino acids phenylalanine, tyrosine and tryptophan. Non-polar regions occur within proteins, membrane lipid bilayers and nucleic acids, e.g. DNA and RNA.
Photosynthesis shows us that quantum coherence occurs among pi resonance rings within such non-polar interiors of proteins at ambient temperatures. Vrtually all biomolecules and organelles have non-polar interiors friendly to quantum coherence (the 'quantum underground').
Consciousness has been proposed to involve organized quantum mechanisms, and in the brain, anesthetic gas molecules selectively prevent consciousness in non-polar, hydrophobic regions of brain proteins. At the turn of the 20th century, Meyer and Overton found that potency of various anesthetic gases correlated precisely with their solubility and binding in a non-polar medium akin to olive oil, later shown to be largely pi electron resonance clouds of 'aromatic' amino acid rings within brain proteins.
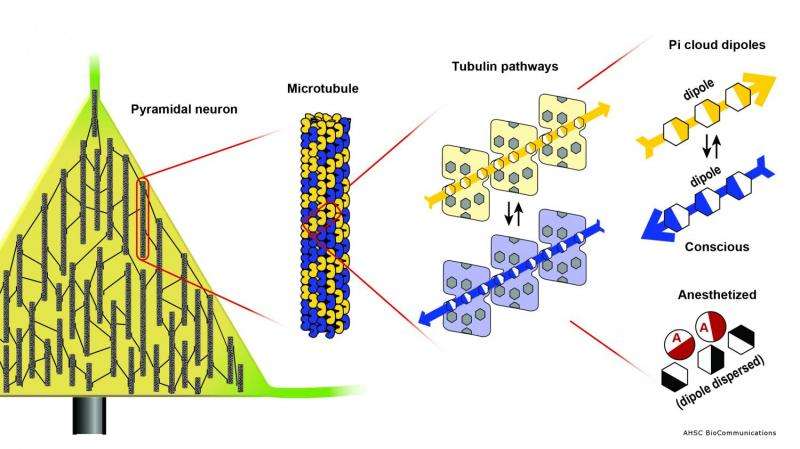
Which proteins? After decades of inconclusive study of membrane receptor proteins, evidence for anesthetic action now points instead to a deeper level inside neurons, in non-polar regions inside the protein walls of cytoskeletal microtubules.
Microtubules are protein lattice polymers which organize neuronal interiors and regulate synapses. Several theories including the Penrose-Hameroff 'Orch OR' theory suggest consciousness depends on microtubules acting as quantum computers whose quantum bits ('qubits') involve coherent dipole couplings among pi electron resonance clouds.
Using molecular modeling, the US-Canadian team of Travis Craddock, Stuart Hameroff and Jack Tuszynski have shown (1) pi resonance rings arrayed in clusters and channels within tubulin, the subunit protein in microtubules, (2) Within these clusters, pi resonance rings are separated by the van der Waals radius, consistent with quantum criticality in the Kauffman/Vattay study, (3) clusters and channels in each tubulin meet those of neighbor tubulins in microtubule lattices, so a quantum underground can extend the length of microtubules, e.g. in helical pathways winding through microtubules, 4) anesthetic molecules bind (Meyer-Overton correlation) and exert their effect in these channels.
What do anesthetics do in the microtubule quantum underground? Quantum coherence in photosynthesis proteins are enabled by coherent mechanical vibrations. In microtubules, Bandyopadhyay's group has shown coherent vibrations in gigahertz (109 Hz), megahertz (106 Hz) and kilohertz (103 Hz) ranges, self-similar patterns each separated by several orders of magnitude. This suggests a scale-invariant hierarchy. Pi resonance rings have coherent resonances in terahertz (1012 Hz) and may be the 'inner apex' of the brain's scale-invariant hierarchy extending through tubuln (gigahertz), microtubules (megahertz), microtubule bundles (kilohertz) and EEG (hertz).
Coherent vibrations enable quantum coherence in photosynthesis proteins. Their role in microtubules may be as important for cognition and consciousness. Microtubule vibrations are accordion-like compressions and relaxations, with each compression pushing the pi resonance rings slightly closer together, past the quantum critical point, beneath the van der Waals radii and enabling quantum coherence throughout large regions of the microtubule quantum underground, and overlapping van der Waals radii, causing nonlinear repulsion and return to classical states. Thus quantum and classical states alternate, in various frequency scales which interact, not unlike music. This solves the problem of having a quantum state isolated from the classical environment, and then being able to communicate with that environment, e.g. for inputs and outputs. Quantum computer pioneer Paul Benioff once wrote about a fictional robot whose quantum computer brain so oscillated between quantum and classical states. Microtubules may vibrate and go in and out of quantum critical range.
Then what do anesthetics do to erase consciousness? Craddock, Hameroff and Tuszynski have modeled dipole-coupled oscillations between benzene ring pi resonance clouds and found intrinsic coherence at a frequency of 68 Terahertz (68 x 1012 Hz). With a nearby anesthetic molecule binding by van der Waals 'dipole dispersion' forces, the energy barrier increases, changing the clocking frequency by 20 percent. Thus anesthesia may disperse dipoles to dampen terahertz vibrations in the quantum underground of brain microtubules. Fortunately, many non-polar regions of the quantum underground in living systems are too small for anesthetic molecules, and so non-conscious quantum coherence continues during anesthesia. Life goes on.
The brain is looking like a scale-invariant hierarchy, with clocking frequencies at different spatio-temporal scales – clocks within clocks within clocks…. Anesthetics act at the deepest level, the fastest clock, the inner apex, in the microtubule quantum underground.
References:
Craddock TJA, Hameroff SR, Tuszynski JA (2015) Anesthetics Act in Quantum Channels in Brain Microtubules to Prevent Consciousness. Current Topics in Medicinal Chemistry 15 (6) 523-533(11)
Hameroff S, Penrose R (2014) Consciousness in the universe – Review of the 'Orch OR' theory. Physics of Life Reviews 11(1):39–78
http://www.sciencedirect.com/science/article/pii/S1571064513001188
Hameroff S, Penrose R (2014) Reply to criticism of the'Orch OR qubit' – Physics of Life Reviews 11(1):104-112 http://www.sciencedirect.com/science/article/pii/S1571064513001917
Hameroff S (2012) How quantum brain biology can rescue conscious free will, Front Integr Neurosci. 6:93. DOI: 10.3389/fnint.2012.00093.
Mudur GS (2015) 'Deep inside cells, a clue to the mind' Calcutta Telegraph www.telegraphindia.com/1150403 … 399.jsp#.VSfJ3EtKh4M
————————
More information: Quantum Criticality at the Origin of Life, arXiv:1502.06880 [cond-mat.dis-nn] arxiv.org/abs/1502.06880
Abstract
Why life persists at the edge of chaos is a question at the very heart of evolution. Here we show that molecules taking part in biochemical processes from small molecules to proteins are critical quantum mechanically. Electronic Hamiltonians of biomolecules are tuned exactly to the critical point of the metal-insulator transition separating the Anderson localized insulator phase from the conducting disordered metal phase. Using tools from Random Matrix Theory we confirm that the energy level statistics of these biomolecules show the universal transitional distribution of the metal-insulator critical point and the wave functions are multifractals in accordance with the theory of Anderson transitions. The findings point to the existence of a universal mechanism of charge transport in living matter. The revealed bio-conductor material is neither a metal nor an insulator but a new quantum critical material which can exist only in highly evolved systems and has unique material properties.
Journal information: Physics of Life Reviews
© 2015 Phys.org