April 9, 2015 report
Measurement of first ionization potential of lawrencium reignites debate over periodic table
(Phys.org)—A team of researchers with member affiliations from across the globe has succeeded in conducting a measurement of the first ionization potential of lawrencium. In their paper published in the journal Nature, the team describes how they achieved the feat and what they believe it means for placement on the Periodic Table of Elements. Andreas Türler of the University of Bern offers a News & Views perspective piece on the work done by the team in the same issue.
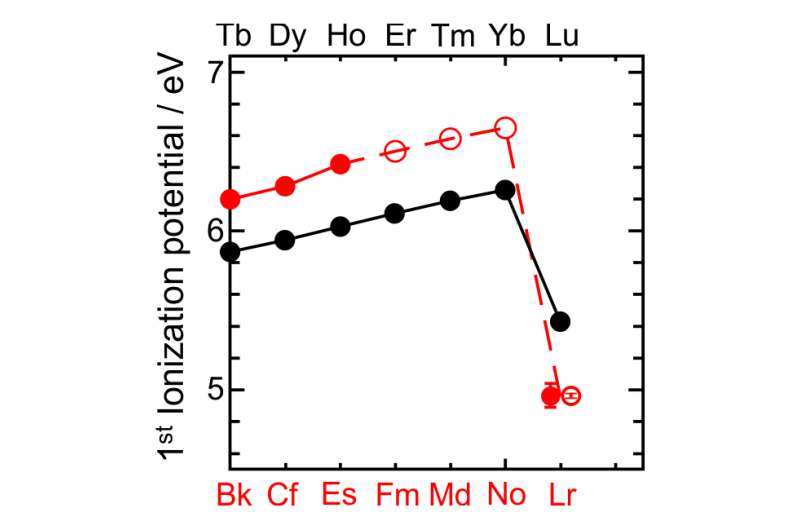
Lawrencium is an element that does not exist in nature, scientists create it in the lab and use it for study, though the process is difficult and the result lasts for only a few seconds. In this new effort, the researchers used a known technique to create the element, then devised a way to measure its first ionization potential—which describes the amount of energy required to cause one atom of it to be turned into an ion by knocking off one of its electrons. It is this measurement that forms the basis of element placement on the periodic table.
To make this measurement for lawrencium (named for Ernest Lawrence), the team created some samples by shooting boron atoms at a bit of californium—doing so caused a few atoms of a lawrencium isotope to come into existence. Those atoms were then captured using a cadmium mist iodide and placed on a piece of metal which was then heated to 2,700 kelvin—hot enough to knock electrons off of some of the atoms. After that, all it took was summing the atoms that were ionized and calculating the energy it took to make it happen. Doing so revealed that it took just 4.96 electronvolts to ionize one of the atoms, an unexpectedly small amount, which likely means that lawrencium's outermost electron is very loosely bound, which means, that placing the element where it has been put on the periodic table up till now, might not work.
As it stands now, the periodic table is arranged in columns and blocks which are based on how an atom's electrons are put together, with different blocks relating to different types of orbital—the shape created by the path of their orbits. Lawrencium, at this time, appears to have a dumb-bell shape. These new findings create conflicting views on where the element should be placed on the table and has reignited debate on the way the table is structured in general.

More information: Measurement of the first ionization potential of lawrencium, element 103, Nature 520, 209–211 (09 April 2015) DOI: 10.1038/nature14342
Abstract
The chemical properties of an element are primarily governed by the configuration of electrons in the valence shell. Relativistic effects influence the electronic structure of heavy elements in the sixth row of the periodic table, and these effects increase dramatically in the seventh row—including the actinides—even affecting ground-state configurations. Atomic s and p1/2 orbitals are stabilized by relativistic effects, whereas p3/2, d and f orbitals are destabilized, so that ground-state configurations of heavy elements may differ from those of lighter elements in the same group. The first ionization potential (IP1) is a measure of the energy required to remove one valence electron from a neutral atom, and is an atomic property that reflects the outermost electronic configuration. Precise and accurate experimental determination of IP1 gives information on the binding energy of valence electrons, and also, therefore, on the degree of relativistic stabilization. However, such measurements are hampered by the difficulty in obtaining the heaviest elements on scales of more than one atom at a time. Here we report that the experimentally obtained IP1 of the heaviest actinide, lawrencium (Lr, atomic number 103), is 4.96 electronvolts. The IP1 of Lr was measured with 256Lr (half-life 27 seconds) using an efficient surface ion-source and a radioisotope detection system coupled to a mass separator. The measured IP1 is in excellent agreement with the value of 4.963(15) electronvolts predicted here by state-of-the-art relativistic calculations. The present work provides a reliable benchmark for theoretical calculations and also opens the way for IP1 measurements of superheavy elements (that is, transactinides) on an atom-at-a-time scale.
Journal information: Nature
© 2015 Phys.org