Detailing heterochromatin formation at the onset of life
Antoine Peters and his group at the Friedrich Miescher Institute for Biomedical Research (FMI) have elucidated the mechanisms controlling the packaging of chromatin in the early embryo. They have identified two molecular pathways that direct heterochromatin formation around centromeres in a manner dependent on the parental origin of the DNA.
To allow it to fit into the nucleus, DNA is tightly wrapped around nuclear proteins. This complex of DNA and proteins, known as chromatin, eventually forms chromosomes. But as well as solving a packaging problem, chromatin substantially influences gene transcription. The more tightly DNA is wound around proteins, the less accessible it is and the less transcription occurs. Understanding the processes that control the compaction of DNA is therefore indispensable for a better understanding of gene regulation and cell fate. This is particularly true in the case of the developing embryo, where crucial cell fate decisions are made from day one onward.
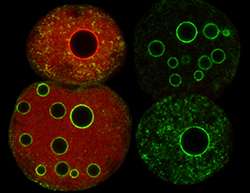
Antoine Peters and his group at the FMI study the mechanisms that regulate chromatin formation in the early embryo. They are particularly interested in the formation and transcriptional regulation of the dense heterochromatin regions flanking the centromeres, which control the equal distribution of DNA to daughter cells during cell division. In the pericentromeric regions, heterochromatin formation can be straightforwardly monitored by microscopy. This facilitated the discovery of the molecular players driving this process. A couple of years ago, Peters' group found to their surprise that, depending on whether the DNA is inherited from the father or the mother, different proteins are involved in heterochromatin formation in these regions.
Now, in a paper published in Molecular Cell, they report the identification of the regulatory factors and mechanisms controlling heterochromatin formation in the maternal and paternal genomes. A key role is played by polycomb repressive complex 1 (PRC1), which localizes to paternal heterochromatin, where it represses transcription. The scientists found that PRC1 is targeted to the paternal genome and excluded from the maternal genome via a mechanism that relies on two chromodomain proteins – Cbx2 and Hp1β – as well as parental specific histone modifications. Peters explains: "PRC1 is directed to the paternal heterochromatin by the chromodomain and neighboring AT-hook of Cbx2, which recognize a particular type of methylated histones and AT rich DNA sequences, respectively. In the maternal situation, on the other hand, pericentromeric regions are marked by another repressive histone methylation, which attracts Hp1β; this, in turn, prevents the association of PRC1 with the genome. Our research explains the differences between pericentromeric heterochromatin formation in the two parental genomes in early embryos. It also elucidates the molecular logic underlying the functional hierarchy between two major chromatin-based repressive pathways."
These findings are noteworthy as they reflect differences in the preparation of chromatin for fertilization during male and female gametogenesis. During the development of the male gamete, the DNA packaging is substantially altered so that the whole genome can fit in the tiny head of the sperm. After fertilization, the packaging of paternal DNA is reprogrammed, in a series of steps, to the canonical form present in oocytes and somatic cell types. As the embryo grows, the differences between maternal and paternal chromatin thus disappear. Peters comments: "We are intrigued by the remarkable diversity in heterochromatin formation at the onset of life. Our current research is focusing on how the mechanisms described may contribute to gene regulation during early embryogenesis."
More information: "Cbx2 targets PRC1 to constitutive heterochromatin in mouse zygotes in a parent-of-origin-dependent manner." Mol Cell, dx.doi.org/10.1016/j.molcel.2015.02.013
Journal information: Molecular Cell