Unlikely hydrogen bond discovered

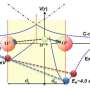
As with magnets and alternating current, positively charged molecules never aim for one another. Indeed, similarly charged poles are repelled. Nevertheless, a team from the University of Copenhagen's Department of Chemistry has managed to become the first to bond positively charged phosphorus atoms with positively charged hydrogen ones. Their insight may prove pivotal to understanding how biologically important molecules such as DNA and proteins form properly.
PhD student Anne Hansen, Post-Doctoral fellow Lin Du and Professor Henrik Kjærgaard discovered the unlikely hydrogen/Phospherous bonds in their shared lab at the University of Copenhagen, physical chemistry section. Their findings were published in the Journal of Physical Chemistry Letters.
Function follows form where proteins are concerned. Whether they serve as signaling agents, catalysts or biological building blocks, proteins are only effective if their molecular structure is spot on. Their composition is largely dependent on hydrogen atoms in the molecules, and the ability of these to create hydrogen bonds with other elements.
Previously, researchers assumed that positively charged hydrogen could only create hydrogen bonds with negatively charged elements like oxygen, fluorine and nitrogen. That positive hydrogen can also be bound to positive phosphorus opens up a world of fresh insight into biological processes. It also provides the basis for an entirely new understanding of how atomic charge works. Thus, it may come as no small surprise that Professor Henrik Kjærgaard is proud of their discovery.
"It was thought that atomic charge was global, that is, as something that was uniform and spherically shaped. But our experiment demonstrates, as clear as day, that charge is asymmetric – that small areas of positive charge exist upon atoms which are in fact negative," explains Kjærgaard.
The discovery was worked on in Professor Kjærgaard's Quantum, Spectra and Dynamics group. The group specializes in combining spectroscopic analyses with theoretical modelling and computational chemistry.
More information: J. Phys. Chem. Lett., 2014, 5 (23), pp 4225–4231 DOI: 10.1021/jz502150d
Journal information: Journal of Physical Chemistry Letters
Provided by University of Copenhagen