February 2, 2015 report
Synthesis and characterization of a hexaarylbene with six unique substituents
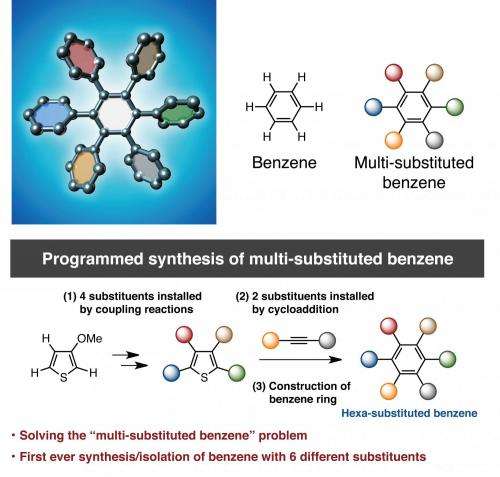
(Phys.org) —The six-carbon aromatic molecule benzene is a staple of organic chemistry. Benzene-derived molecules have applications that include everything from pharmaceuticals to nano devices. However, one of the difficulties with making benzene-derived compounds is controlling how the substituents are placed around the benzene ring.
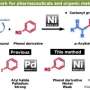
Kenichiro Itami and Junichiro Yamaguchi of Nagoya University present, for the first time, a generally applicable synthesis that yields hexaarylbenzene molecules with five and six different aromatic substituents replacing the hydrogens on a benzene ring. Their synthesis is reported in Nature Chemistry.
One of the difficulties in adding aryl substituents to a benzene ring is that traditional reactions tend to add substituents symmetrically. Attempts to produce benzene-derived molecules with four or more unique aryl substituents usually produce several isomers that are difficult to isolate.
Recent research in benzene chemistry has expanded the repertoire of reactions and possible substituents. However, these reactions often require costly catalysts and starting materials, and are too specific to be generalized for a variety of substituents and core aromatic compounds. Additionally, current methods have only been able to accomplish benzene-derived molecules with three or four different aryl groups that are selectively substituted at particular positions on the benzene ring.
Ideally, if a generally applicable reaction could be found that allowed for complete control over replacing the benzene hydrogens with diverse aryl molecules, this would open the door for making a variety of hexaarylbenzene molecules for a wide range of applications. Itami and Yamaguchi sought to design this kind of reaction by modifying some of their prior studies using 3-methoxythiophene as the starting material for making benzenes with five or six different aromatic substituents.
The first step in the process was to make a tetraarylthiophene with four of the aromatic substituents that they wanted to place on the benzene ring. Itami and Yamaguchi were able to make the target tetraarylthiophene isometrically pure and in sufficient amount. However, they found that they needed to convert the thiophene to a thiophene S-oxide in order to complete the [4+2] cycloaddition reaction necessary to form the target hexaarylbenzene. After making this modification, they were able to isolate an isometetrically pure hexaarylbenzene with five different substituents.
They were eventually able to use the same reaction procedure to make a hexaarylbenzene with six different substituents by changing one of the aryl groups on the diarylethyne used in the [4+2] cycloaddition reaction. This reaction, however, yielded a product with two isomers. They were able to obtain isomerically pure crystals of the target hexaarylbenzene after making chemical modifications to the mixed product.
This is the first time that a reaction mechanism has been reported that produces a hexaarylbenzene with six different substituents. Itami and Yamaguchi were able to isolate pure crystals, characterize their product, and conduct preliminary fluorescence and absorption studies.
Additionally, Itami and Yamaguchi tested the reactivity and versatility of their synthetic method using tetraarylnapthalene- and pentaarylpyridine-derived compounds. They used the same reaction procedure, starting with tetraarylthiophene, and made compounds with multiple different aryl substituents.
Itami and Yamaguchi point out that there are still some improvements to their reaction procedure that need to be made for benzene substituion to be "truly programmable," including higher product yields for their synthesis, and more efficient ways to produce hexaarylbenzenes that are isometrically pure.
More information: Synthesis and characterization of hexaarylbenzenes with five or six different substituents enabled by programmed synthesis, Nature Chemistry (2015) DOI: 10.1038/nchem.2174
Abstract
Since its discovery in 1825, benzene has served as one of the most used and indispensable building blocks of chemical compounds, ranging from pharmaceuticals and agrochemicals to plastics and those used in organic electronic devices. Benzene has six hydrogen atoms that can each be replaced by different substituents, which means that the structural diversity of benzene derivatives is intrinsically extraordinary. The number of possible substituted benzenes from n different substituents is (2n + 2n2 + 4n3 + 3n4 + n6)/12. However, owing to a lack of general synthetic methods for making multisubstituted benzenes, this potentially huge structural diversity has not been fully exploited. Here, we describe a programmed synthesis of hexaarylbenzenes using C–H activation, cross-coupling and [4+2] cycloaddition reactions. The present method allows for the isolation and structure–property characterization of hexaarylbenzenes with distinctive aryl substituents at all positions for the first time. Moreover, the established protocol can be applied to the synthesis of tetraarylnaphthalenes and pentaarylpyridines.
Journal information: Nature Chemistry
© 2015 Phys.org