Where did all the xenon go?
(Phys.org) —The noble gas xenon should be found in terrestrial and Martian atmospheres, but researchers have had a hard time finding it.
The prevailing theory claims that due to xenon's weight—it is a heavy gas—it could be trapped in a planet's core or in the mantle during the planet's formation.
Lawrence Livermore scientists and collaborators have discovered that the xenon can be trapped in the subsurface of the Earth, shedding new insights into the long-standing mysteries of the "missing xenon" in earth science.
The discovery of the noble gas xenon (Xe) has led to the synthesis of hundreds of Xe compounds (for example, it is thought that a compound made up of xenon and iron may lie in Earth's core). Its reactivity also has been estimated to be the cause of its depletion by a factor of 20 relative to the lighter noble gases—neon, argon and krypton—in the atmosphere of Earth, Mars and other planetary bodies. Specifically, xenon reacts with hydrogen and ice at high pressures to form stable compounds.
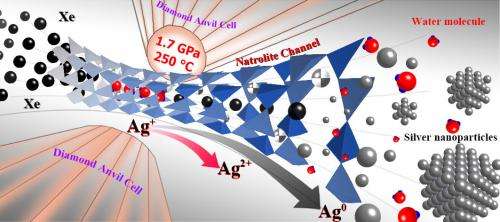
The team used a high pressure diamond anvil cell, which applies extreme pressures on materials, and advanced synchrotron X-ray scattering techniques to show that under high pressure and temperature, a silicate mineral, made up mostly of silver, irreversibly inserts xenon into its micropores and undergoes charge separation. As opposed to other noble gases such as argon and krypton, xenon stays within the pores even after pressure and heat are decreased.
"This is a new chemical reaction that could account for the 'missing xenon' observed in terrestrial and Martian atmospheres," said Hyunchae Cynn, one of the LLNL physicists involved in the research. The team found missing xenon from the atmosphere trapped within porous rocks in a planet's core or mantle.
In the experiments, the temperatures and pressures used were within the range of hydrothermal conditions found in subduction zones on Earth and the subsurface of other planetary bodies such as Mars. Cynn suggests that the noble gas chemistry in Mars may have a similar missing xenon signature like Earth does and may help explain Xe transport and subsurface trapping.
The research appears in the September issue of the journal Nature Chemistry.
More information: Nature Chemistry, www.nature.com/nchem/journal/v … full/nchem.1997.html
Journal information: Nature Chemistry
Provided by Lawrence Livermore National Laboratory