Fundamental change in the nature of chemical bonding upon isotopic substitution
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. Because of the resulting differences in mass ratios, isotope effects can significantly influence the formation and breaking of chemical bonds in the course of chemical reactions. In extreme cases, isotopic substitution can even cause a fundamental change in the type of chemical bond, as reported by scientists in the journal Angewandte Chemie.
Such effects come to light most distinctly when the differences between the isotopes are particularly large, like those of hydrogen. In nature, hydrogen is found as 1H (protium, H), 2H (deuterium, D), and 3H (tritium, T). Additional isotopes have been synthesized in the lab, including some exotic atoms that contain other elementary particles. For example, muonium (symbol Mu) is made of an electron and a nucleus consisting of an antimuon. Chemically, it behaves like a hydrogen isotope, but it is nine times lighter than 1H.
Jörn Manz (Shanxi University Taiyuan and FU Berlin), Donald G. Fleming (University of British Columbia, Canada), Kazuma Sato, and Toshiyuki Takayanagi (Saitama University, Japan) have carried out novel quantum-mechanical ab initio calculations for the reaction between hydrogen bromide and bromine atoms to form the BrHBr radical. They were particularly interested in a comparison between the hydrogen isotopes H, D, T, and Mu, as well as a heavy exotic isotope. "We found that the four heavy isotopomers behave essentially the same way," says Manz. " In contrast, the lightest isotopomer, BrMuBr, is held together by a completely different kind of chemical bond."
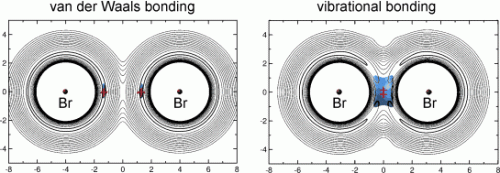
BrHBr and its heavy analogues can adopt either a linear or a bent form. In the bent form, both bromine atoms are bound to each other. In the linear form, the two bromine atoms are bonded by way of the H atom, which is positioned very close to one of the two Br atoms while remaining significantly farther away from the other. Van der Waals bonds hold the whole thing together. These result from short-term charge transfers within a particle, which have an attractive effect.
In contrast, BrMuBr is bound together by a recently proposed, completely different type of bonding: vibrational bonding. In this type of bond, the molecular fragments are held together by their motion: the muonium vibrates in a transition state between the two bromine atoms. "Our calculations on BrMuBr are the first clear evidence for the existence of this new type of bonding," says Manz. "In addition, they are the first indication that isotopic substitution can change the nature of chemical bonding in a profound manner. The different isotopomers of the radical we have studied and compared here have completely different structures, symmetries, and, most importantly, energetics and mechanisms of chemical bonding."
More information: Fleming, D. G., Manz, J., Sato, K. and Takayanagi, T. (2014), "Fundamental Change in the Nature of Chemical Bonding by Isotopic Substitution." Angew. Chem. Int. Ed.. doi: 10.1002/anie.201408211
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Angewandte Chemie




















