Thermoset materials acquire thermoplastic properties with the aid of triazolinediones
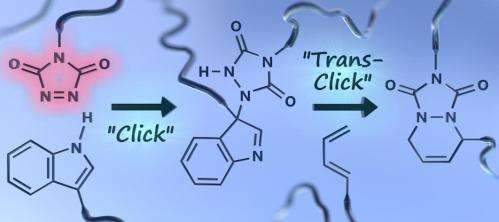
At Ghent University (Belgium), a new type of so-called 'click' chemistry has been introduced. Like with most of click chemistry, it is based on a long-known efficient chemical reaction, which was now also found to be very practical for diverse and demanding applications. In particular, the unique reactivity of the studied 1,2,4-triazoline-3,5-dione (TAD) reagents has been harnessed to reversibly crosslink polyurethanes, or almost any other polymer matrix. At higher temperatures the TAD-induced crosslinks can temporarily open up, giving the thermoset the ability to be reshaped or even extruded like a typical thermoplastic polymer. This is reported in the current issue of Nature Chemistry, in a joint paper by three Ghent research groups led by Filip Du Prez, Veronique Van Speybroeck and Johan Winne.
The TAD reagents used in these new click reactions are quite stable compounds in bulk, but react almost instantaneously with electron rich unsaturated organic compounds. These preferred and orthogonal reaction partners can be easily implemented in polymer chains, resulting in very straightforward macromolecular linking and functionalisation. The TAD-reagents can be clicked to these reaction partners at room temperature without the aid of any catalyst in an almost instantaneous and air- and moisture insensitive manner. In most cases, this 'dummy proof/sure thing'-reaction will lead to the creation of an irreversible chemical bond. But when the TAD-reaction partner is part of an indole group, a temporary 'unclick reaction' can happen at temperatures around 120°C, releasing the original TAD reagent, which can then click to another partner.
With this TAD-based chemistry, a series of click reactions can thus be performed with alternating reaction partners. The reaction sequence can even be 'programmed' by choosing suitable alternative substrates. This is a new concept for the field, which was named a 'transclick' reaction by the Ghent researchers.
The key feature of the TAD reagents, which enables these new 'dynamic' click chemistry applications, is the remarkable reaction kinetics. The energy barriers that are associated with the bond-forming and –breaking steps are very low. This has been verified by advanced calculations. The usual way to 'make a reaction click' is to make sure it is a very energetically favorable reaction. This implies that the backward reaction is highly unfavorable, and thus not feasible. "In our search for a suitable reaction partner for ultrafast reacting TADs, all we needed to do was make sure that the reaction was favorable enough to be a true click reaction under ambient conditions, while retaining the ability to reverse the process at a reasonable temperature," the researchers explain.
What results is a very user-friendly chemical system for performing click reactions. This fact can be further appreciated by another useful feature of the process: TAD molecules are bright red compounds, which are rendered completely colorless after reaction, giving the user an immediate visual feedback. Additionally, the fact that most indole and TAD molecules can be synthesized from bulk chemicals and thus on a large scale, makes this a very interesting platform for industrial partners. The unique reactivity of TAD also opens up possibilities to functionalise or cross-link simple but unreactive raw materials such as natural plant oils. "This is the first time our research group has been spontaneously contacted by several (chemical) companies, interested in performing joint research on one particular type of chemistry for so many different types of applications", Prof. Du Prez clarifies.
More information: "Triazolinediones enable ultrafast and reversible click chemistry for the design of dynamic polymer systems." Stijn Billiet,et al.Nature Chemistry 6, 815–821 (2014) DOI: 10.1038/nchem.2023. Received 16 February 2014 Accepted 26 June 2014 Published online 11 August 2014 Corrected online 11 August 2014
Journal information: Nature Chemistry
Provided by Ghent University


















