Study shows calcium carbonate takes multiple, simultaneous roads to different minerals

One of the most important molecules on earth, calcium carbonate crystallizes into chalk, shells and minerals the world over. In a study led by the Department of Energy's Pacific Northwest National Laboratory, researchers used a powerful microscope that allows them to see the birth of crystals in real time, giving them a peek at how different calcium carbonate crystals form, they report in September 5 issue of Science.
The results might help scientists understand how to lock carbon dioxide out of the atmosphere as well as how to better reconstruct ancient climates.
"Carbonates are most important for what they represent, interactions between biology and Earth," said lead researcher James De Yoreo, a materials scientist at PNNL. "For a decade, we've been studying the formation pathways of carbonates using high-powered microscopes, but we hadn't had the tools to watch the crystals form in real time. Now we know the pathways are far more complicated than envisioned in the models established in the twentieth century."
Earth's Reserve
Calcium carbonate is the largest reservoir of carbon on the planet. It is found in rocks the world over, shells of both land- and water-dwelling creatures, and pearls, coral, marble and limestone. When carbon resides within calcium carbonate, it is not hanging out in the atmosphere as carbon dioxide, warming the world. Understanding how calcium carbonate turns into various minerals could help scientists control its formation to keep carbon dioxide from getting into the atmosphere.
Calcium carbonate deposits also contain a record of Earth's history. Researchers reconstructing ancient climates delve into the mineral for a record of temperature and atmospheric composition, environmental conditions and the state of the ocean at the time those minerals formed. A better understanding of its formation pathways will likely provide insights into those events.
To get a handle on mineral formation, researchers at PNNL, the University of California, Berkeley, and Lawrence Berkeley National Laboratory examined the earliest step to becoming a mineral, called nucleation. In nucleation, molecules assemble into a tiny crystal that then grows with great speed. Nucleation has been difficult to study because it happens suddenly and unpredictably, so the scientists needed a microscope that could watch the process in real time.
Come to Order
In the 20th century, researchers established a theory that crystals formed in an orderly fashion. Once the ordered nucleus formed, more molecules added to the crystal, growing the mineral but not changing its structure. Recently, however, scientists have wondered if the process might be more complicated, with other things contributing to mineral formation. For example, in previous experiments they've seen forms of calcium carbonate that appear to be dense liquids that could be sources for minerals.
Researchers have also wondered if calcite forms from less stable varieties or directly from calcium and carbonate dissolved in the liquid. Aragonite and vaterite are calcium carbonate minerals with slightly different crystal architectures than calcite and could represent a step in calcite's formation. The fourth form called amorphous calcium carbonate—or ACC, which could be liquid or solid, might also be a reservoir for sprouting minerals.
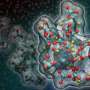
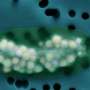
To find out, the team created a miniature lab under a transmission electron microscope at the Molecular Foundry, a DOE Office of Science User Facility at LBNL. In this miniature lab, they mixed sodium bicarbonate (used to make club soda) and calcium chloride (similar to table salt) in water. At high enough concentrations, crystals grew. Videos of nucleating and growing crystals recorded what happened [URLs to come].
Morphing Minerals
The videos revealed that mineral growth took many pathways. Some crystals formed through a two-step process. For example, droplet-like particles of ACC formed, then crystals of aragonite or vaterite appeared on the surface of the droplets. As the new crystals formed, they consumed the calcium carbonate within the drop on which they nucleated.
Other crystals formed directly from the solution, appearing by themselves far away from any ACC particles. Multiple forms often nucleated in a single experiment—at least one calcite crystal formed on top of an aragonite crystal while vaterite crystals grew nearby.
What the team didn't see in and among the many options, however, was calcite forming from ACC even though researchers widely expect it to happen. Whether that means it never does, De Yoreo can't say for certain. But after looking at hundreds of nucleation events, he said it is a very unlikely event.
"This is the first time we have directly visualized the formation process," said De Yoreo. "We observed many pathways happening simultaneously. And they happened randomly. We were never able to predict what was going to come up next. In order to control the process, we'd need to introduce some kind of template that can direct which crystal forms and where."
In future work, De Yoreo and colleagues plan to investigate how living organisms control the nucleation process to build their shells and pearls. Biological organisms keep a store of mineral components in their cells and have evolved ways to make nucleation happen when and where needed. The team is curious to know how they use cellular molecules to achieve this control.
More information: Michael H. Nielsen, Shaul Aloni, and James J. De Yoreo. In Situ TEM Imaging of CaCO3 Nucleation Reveals Coexistence of Direct and Indirect Pathways, Science September 5, 2014, DOI: 10.1126/science.1254051
Journal information: Science
Provided by Pacific Northwest National Laboratory