Understanding mussels' stickiness could lead to better surgical and underwater glues
Mussels might be a welcome addition to a hearty seafood stew, but their notorious ability to attach themselves to ships' hulls, as well as to piers and moorings, makes them an unwelcome sight and smell for boaters and swimmers. Now, researchers report in ACS' journal Langmuir a clearer understanding of how mussels stick to surfaces, which could lead to new classes of adhesives that will work underwater and even inside the body.
Shabeer Ahmad Mian and colleagues note that mussels have a remarkable knack for clinging onto solid surfaces underwater. That can make them a real nuisance to recreational boaters and professional fishermen, who have to scrape the hitchhikers off their vessels to help them run more efficiently. Some types of mussels can even plug up drinking water pipes. Mussels also can stick to materials with nonstick coatings. Although researchers have already developed mussel-inspired glues, they still don't have a full understanding of exactly how these critters stick so well to underwater surfaces. So, Mian's team set out to investigate this mystery in painstaking detail to improve these adhesives and to develop new ones.
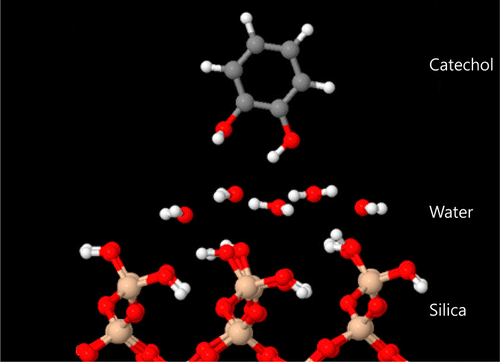
Using complex calculations and simulations, they determined that one part of the mussel "glue" molecule, called catechol, pushes water molecules out of the way to bind directly to wide variety of surfaces. They say that this study provides a clear picture of the first step of mussel adhesion, which could pave the way for better adhesives for many applications, such as for use in surgeries. The adhesives can be nontoxic and biocompatible, says Mian.
More information: "A Fundamental Understanding of Catechol and Water Adsorption on a Hydrophilic Silica Surface: Exploring the Underwater Adhesion Mechanism of Mussels on an Atomic Scale" Langmuir, Article ASAP. DOI: 10.1021/la500800f
Abstract
Mussels have a remarkable ability to bond to solid surfaces under water. From a microscopic perspective, the first step of this process is the adsorption of dopa molecules to the solid surface. In fact, it is the catechol part of the dopa molecule that is interacting with the surface. These molecules are able to make reversible bonds to a wide range of materials, even underwater. Previous experimental and theoretical efforts have produced only a limited understanding of the mechanism and quantitative details of the competitive adsorption of catechol and water on hydrophilic silica surfaces. In this work, we uncover the nature of this competitive absorption by atomic scale modeling of water and catechol adsorbed at the geminal (001) silica surface using density functional theory calculations. We find that catechol molecules displace preadsorbed water molecules and bond directly on the silica surface. Using molecular dynamics simulations, we observe this process in detail. We also calculate the interaction force as a function of distance, and observe a maximum of 0.5 nN of attraction. The catechol has a binding energy of 23 kcal/mol onto the silica surface with adsorbed water molecules.
Journal information: Langmuir
Provided by American Chemical Society