Mammalian cells engineered to produce longer-lived versions of therapeutic protein erythropoietin
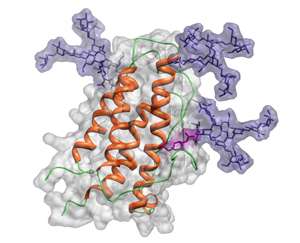
Erythropoietin (EPO)—a key regulator of red blood cell production—is widely used for treating certain cancers and anemia induced by chronic kidney disease. However, the time that EPO resides in the bloodstream depends on the extent to which it is modified by sugar chains containing sialic acid. This modification, known as sialylation, prevents liver cells from taking up and destroying glycosylated EPO (see image). EPO fully modified with sugars containing sialic acid persists in the bloodstream for about one hour—five times longer than unsialylated EPO.
Since as much as 80% of manufactured EPO is discarded because it is insufficiently sialylated, optimizing the sialylation of EPO may reduce the expense and dosing requirements of EPO therapy.
Previously, a team led by Zhiwei Song of the A*STAR Bioprocessing Technology Institute in Singapore increased the sialylation level of EPO in mammalian cells deficient in the enzyme N-acetylglucosaminyltransferase (GnT I) by introducing a functional GnT I gene to the cell line. EPO sialylation in the genetically modified cells was almost 25% more than that obtained from wild-type cells when cultured under laboratory conditions.
The same group has now improved on the capacity of that line by using a process called methotrexate amplification to boost yields of sialylated EPO by an additional 2.5-fold. Moreover, the increased yields can be achieved under industrially relevant conditions.
The process involves inactivating the gene that normally makes dihydrofolate reductase (DHFR) in the GnT I-deficient cell line, and then expressing a segment of foreign DNA that encodes EPO, DHFR and a foreign GnT I enzyme. Because methotrexate inhibits DHFR, which is essential for cell survival, treatment of this modified cell line with increasing concentrations of methotrexate selects cells that have multiple copies of the DHFR gene on the foreign DNA segment. As these cells also have multiple copies of the adjacent EPO and GnT I genes, the process also boosts levels of fully sialylated EPO.
The researchers tested the sialic acid content of the EPO produced from the best line and found that it was over 60% higher than the amount produced by an existing industrial line. Although the yields obtained are still not high enough for manufacturing industrially relevant levels of EPO, Song is confident that additional rounds of methotrexate amplification and modification of the medium and other culture conditions could further increase yields. "Optimal glycosylation is an important consideration for the regulatory use of many protein drugs," he adds. "So our strategy might find applications beyond EPO alone."
More information: Goh, J. S. Y., Liu, Y., Liu, H., Chan, K. F., Wan, C. et al. Highly sialylated recombinant human erythropoietin production in large-scale perfusion bioreactor utilizing CHO-gmt4 (JW152) with restored GnT I function. Biotechnology Journal 9, 100–109 (2014). dx.doi.org/10.1002/biot.201300301