A bacterial ballistic system
Many pathogenic bacteria use special secretion systems to deliver toxic proteins into host cells. Researchers of Ludwig-Maximilians-Universitaet (LMU) in Munich have determined the structure of a crucial part of one of these systems – which are possible targets for novel antibiotics.
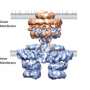
Bacteria secrete a broad range of specific proteins that can affect the behavior or survival of cells in their environment. Among the specialized transport systems responsible for the export of such factors are so-called Type VI secretion systems. In collaboration with Axel Mogk of the Center for Molecular Biology Heidelberg (ZMBH), biochemist Petra Wendler at the LMU's Gene Center has now determined the three-dimensional structure of one of these export complexes. "Bacterial species employ these systems primarily to secrete specific toxic proteins directed against competitors or host cells. The protein complexes involved in secretion essentially function as nanosyringes," says Wendler.
Type VI secretion systems were discovered only a few years ago, but they are synthesized by many bacterial species, including important pathogens, such as Vibrio cholerae, the bacterium that causes cholera, and Pseudomonas aeruginosa which can induce severe lung damage during chronic infections. While antibiotic resistance continues to rise, there is a pressing need for alternative ways to combat bacterial pathogens. In this context, Type VI secretion systems offer an interesting target, as blocking their function would effectively disarm pathogenic bacteria in a highly specific manner.
The contractile sheath
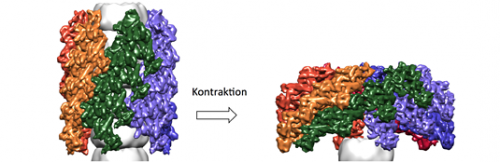
"However, in order to identify weak points in this bacterial secretion system, we need to obtain further insights into how its export machinery works," says Wendler. "A tubular protein complex was recently characterized, which contracts to expel toxins from the cell. Besides characterizing a possible target for novel antibiotics, we were particularly interested in elucidating the mechanism of contraction of the complex, which does not require input of metabolic energy." To answer both of these questions, it is essential to obtain a more detailed picture of the structure of the complex – and Wendler and her associates have taken a significant step in that direction.
"We were able to determine the structure of the contracted form of the outer sheath complex at sub-nanometer resolution. It turns out that its basic architecture and the structural elements that stabilize the complex are related to those of certain proteins found in bacterial viruses, but have been modified in the course of evolution to enable them to serve a secretory function," as Wendler explains. Notably, in the contracted form of the complex studied by Wendler and her colleagues, the recognition site for ClpV, a protein that recycles the subunits of the tubular complex after ejection of effector proteins, is exposed and available for binding. In contrast, in a model of the extended configuration, this site is inaccessible. This neatly explains why the sheath is disassembled only after the toxins have been injected into the target cell.
Wendler and her team now plan to improve the resolution of the structural data still further, and to characterize in detail the conformation of the contractile apparatus in its extended state, so to gain insight into the mechanism responsible for the contraction process itself. "A comprehensive understanding of the structure and function of all the components of this molecular machine could facilitate the development of new, effective antibiotics," Wendler concludes.
More information: www.cell.com/cell-reports/abst … 2211-1247(14)00428-8
Provided by Ludwig Maximilian University of Munich