New perspectives to the design of molecular cages
Researchers from the University of Jyväskylä report a new method of building molecular cages. The method involves the exploitation of intermolecular steric effects to control the outcome of a self-assembly reaction.
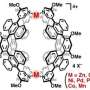
Molecular cages are composed of organic molecules (ligands) which are bound to metal ions during a self-assembly process. Depending on the prevailing conditions, self-assembly processes urge to maximize the symmetry of the system and thus occupy every required metal binding site. The research group led by docent Manu Lahtinen (University of Jyväskylä, Department of Chemistry) developed a method in which sterically hindered ligands are used to seemingly disrupt the self-assembly process. This new strategy allows a ligand to occupy only two of the four potential binding sites of the metal. The created molecular cage presents a low symmetric tetrahedral intermediate product in a reaction that would generally yield a higher symmetric octahedral cage.
The reported results provide new insights on how self-assembly processes of metal-organic systems can be controlled. However, the most significant feature of the proposed method is the new way of building molecular cages with vacant metal binding sites. This creates an opportunity to modify the properties and behavior of pre-assembled cages by incorporating functionally significant molecules to partially exposed metal ions. The presented strategy provides a new concept to build more complex molecular cages.
Molecular cages are the materials of tomorrow
Molecular cages and capsules are hollow nano-sized (1 x 10-9 m) compounds that consist of organic molecules or ions and, in most cases, metal ions. They share many structural features with, for example, viruses whose shells (capsids) are composed of organized proteins. One of the most significant feature of molecular cages is their ability to bind and release guest molecules depending on the prevailing conditions. Hence, some of their most important potential applications include biomedicinal uses (transport of drugs), storing of unstable and/or reactive molecules and recovery of hazardous compounds from aquatic environment.
The present study was published in the journal Chemical Communications and is part of M.Sc. Anssi Peuronen's doctoral thesis that focuses on the study of structural chemistry of cationic ammonium compounds and is supervised by Docent Manu Lahtinen. The third member of the research group is M.Sc. student Samu Forsblom.
More information: Anssi Peuronen, Samu Forsblom and Manu Lahtinen: "Sterically controlled self-assembly of tetrahedral M6L4 cages via cationic N-donor ligands," Chem. Commun., 2014, 50, 5469 - 5472, dx.doi.org/10.1039/C3CC49663E
Journal information: Chemical Communications
Provided by Academy of Finland