Taming a poison: Saving plants from cyanide with carbon dioxide
A team of Canadian and Finnish scientists has discovered cyanoformate—a simple, unstable ion involved in the fruit-ripening process that has evaded detection for decades. Their findings reveal that the surrounding medium greatly impacts the stability of cyanoformate. While this allows carbon dioxide to deactivate cyanide's killer capabilities in fruit, recognizing the factors governing cyanoformate's instability has a larger implication: understanding low-energy carbon dioxide 'catch-and-release.'
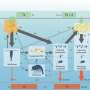
The scientific world is one step closer to understanding how nature uses carbon-capture to tame poisons, thanks to a recent discovery of cyanoformate by researchers at Saint Mary's University (Halifax, Canada) and the University of Jyväskylä (Finland). This simple ion—which is formed when cyanide bonds to carbon dioxide—is a by-product of the fruit-ripening process that has evaded detection for decades.
Chemists have long understood the roles presence of cyanide (CN−) and carbon dioxide (CO2) in fruit ripening, but have always observed them independently. This is the first time scientists have isolated the elusive cyanoformate anion (NCCO2−) and characterized its structure using crystallography and computational chemistry.
The results of the two-year study led by Dr. Jason Clyburne, Saint Mary's University, and Dr. Heikki M. Tuononen, University of Jyväskylä, were released today in the journal Science.
Their findings demonstrate the profound effect the surrounding medium has on the stability of cyanoformate. This situational stability allows carbon dioxide to deactivate cyanide's toxic capabilities at the enzyme's active site where chemical reactions take place. Subsequently, the cyanoformate migrates to the cytoplasm of the cell where it breaks down, releasing the toxic cyanide at a location where it can be dealt with. While this explains how the formation of cyanide does not halt the fruit ripening process, the implications extend far beyond plants and a single enzyme. Recognizing the factors governing the stability of cyanoformate furthers our understanding of carbon-capture, a process used to trap and store carbon dioxide in the environment.
"Here we have a perfect example of nature taming a poison, and what better way to learn the chemistry of carbon-capture than from nature itself?" says Dr. Jason Clyburne, Canada Research Chair in Environmental Science and Materials, and professor of Environmental Science and Chemistry at Saint Mary's University.
"One of the biggest challenges in carbon capture is finding a material that not only captures CO2, but easily releases it," says Luke Murphy, a Masters of Science candidate at Saint Mary's who prepared the crystalline material for the study. "Cyanoformate does both and can be used as a model to develop a greener alternative."
This discovery highlights the importance of applied chemistry to other areas of science and indicates there is still more to be learned about the chemistry of carbon dioxide in cells.
"The fact that cyanoformate was undetected for so long begs the question: What other simple chemistry have we missed?" asks Dr. Heikki M. Tuononen, Academy of Finland research fellow, and senior lecturer at University of Jyväskylä, Finland.
More information: "A Simple Complex on the Verge of Breakdown: Isolation of the Elusive Cyanoformate Ion" Science, 2014.
Journal information: Science
Provided by Saint Mary's University