Steering chemical reactions with laser pulses
With ultra-short laser pulses, chemical reactions can be controlled at the Vienna University of Technology. Electrons have little mass and are therefore influenced by the laser, whereas the atomic nuclei are much heavier and are hardly affected.
Usually, chemical reactions just take their course, much like a ball rolling downhill. However, it is also possible to deliberately control chemical reactions: at the Vienna University of Technology, molecules are hit with femtosecond laser pulses, changing the distribution of electrons in the molecule. This interaction is so short that at first it does not have any discernable influence on the atomic nuclei, which have much more mass than the electrons. However, the disturbance of the electron distribution can still initiate chemical processes and eventually separate the nuclei from each other. The properties of the laser pulse determine which chemical final products are created.
Controlling Chemistry
Chemists can choose which molecules they want to take part in a chemical reaction – but the result is usually determined by the physical and chemical properties of molecules and by external parameters such as the temperature. The reaction itself cannot be controlled. Researchers at the Vienna University of Technology (Photonics Institute) have now succeeded in directly inducing the splitting of hydrocarbons such as ethylene (C2H4) or acetlyene (C2H2) into smaller fragments.
"We are using two different laser pulses", says Markus Kitzler (TU Vienna). "The first pulse takes about 50 femtoseconds and makes the molecules rotate at different speeds." After some time, all molecules are approximately aligned - and then the second laser pulse is applied, which only lasts for five femtoseconds, less than two oscillations of the light wave. This pulse changes the state of the electrons; it can even remove electrons from the molecule.
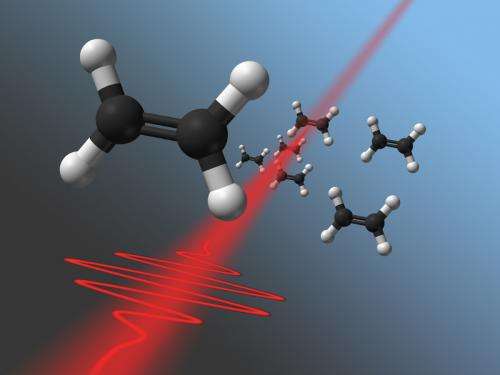
Selecting a Reaction Path
Electrons weigh much less than atomic nuclei. Therefore the electrons can be influenced dramatically by the laser pulse, whereas the heavier nuclei are much too inert for any observable motion in this short period of time. If, however, exactly the right electrons are removed from the molecule, the molecule can be made to break at a specific position. That way, acetylene (C2H2) can be broken up into CH2+, CH+, or carbon ions (C+). "Various reaction paths are possible. For the first time, we managed to distinguish these paths and select the reaction we want", says Markus Kitzler.
An extremely short laser pulse – five femtoseconds (5.10^(-15) seconds) are just five millionths of a billionth of a second – initiates a chemical process, which takes place on a much larger timescale. This is similar to a short explosion at precisely the right places, which may cause a huge building to sway and eventually collapse.
The composition of chemical end products can be controlled by a number of different parameters: The alignment of the molecules by the first laser pulse, the duration and the intensity of the second pulse, which ionizes the molecules.
The experiments were done by Markus Kitzler's research team, his postdoc Xinhua Xie played a leading role in data analysis. Katharinia Doblhoff-Dier and Prof. Stefanie Gräfe from Jena University and Erik Lötstedt, from Tokyo University contributed model calculations, which were invaluable for the interpretation of the experimental results.
The experimental results have now been published in two scientific publications: In Physical Review Letters and Physical Review X.
More information: "Electronic Predetermination of Ethylene Fragmentation Dynamics." Xinhua Xie, Stefan Roither, Markus Schöffler, Erik Lötstedt, Daniil Kartashov, Li Zhang, Gerhard G. Paulus, Atsushi Iwasaki, Andrius Baltuška, Kaoru Yamanouchi, and Markus Kitzler. Phys. Rev. X 4, 021005 – Published 7 April 2014. journals.aps.org/prx/abstract/ … 03/PhysRevX.4.021005
Journal information: Physical Review Letters , Physical Review X
Provided by Vienna University of Technology



















