Building the ring to divide them all: Septin proteins bundle actin filaments into a ring
Researchers of the FOM Institute AMOLF and from Marseille and Paris have demonstrated that cells require a protein called septin to build a ring of actin filaments. This ring of proteins is crucial to drive cell division. The finding explains cell division defects associated with mutations in septin. On March 16, the results of this research were published as advanced online publication in Nature Cell Biology.
Using live-cell microscopy the researchers showed that fruit fly embryos lacking septin could no longer build tightly packed contractile rings. In a follow-up step, the researchers purified the septin protein and showed in test tube experiments how septin bundles cellular actin filaments into rings.
The ring that divides them all
Animal cell division requires physical constriction of the cell membrane into two daughter cells. Constriction is powered by a contractile ring of circumferential actin filaments that is actively contracted by myosin motor proteins.
Even though the principal components of this contractile ring are known, it is unclear how the ring assembles, and in particular how the stiff actin filaments are bent into a highly curved ring. The researchers of the group of Prof.dr. Gijsje Koenderink and the research group in Marseille hypothesized that the protein septin plays a crucial role in ring assembly. Septins are present in the contractile ring of dividing cells in organisms ranging from simple unicellular yeast cells all the way to man. Septin deficiency is known to cause defects in cell division.
Cell divisions in fruit fly embryos
To find out how septins contribute to the assembly of contractile rings, the researchers studied fruit fly embryos. Fruit fly embryos are a convenient model system because one can observe many contractile rings of one embryo at the same time, thus obtaining good statistics.
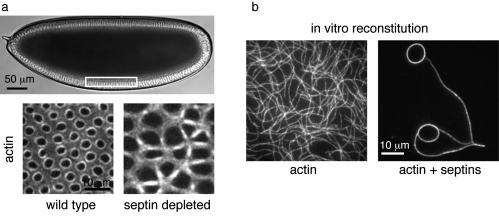
About four hours after fertilization, fly embryos are ellipsoidal and consist of a single cytoplasm with about six thousand nuclei underneath the cell membrane. In a remarkable process known as cellularization, the cell membrane invaginates between all the nuclei and a contractile ring of actin and myosin assembles at each invaginating membrane front. The rings constrict and eventually close off all the invaginated membranes, thus forming a monolayer of epithelial cells.
To pinpoint the role of septins, the researchers in Marseille performed live cell imaging of wild-type (normal) embryos and genetically modified embryos that lack septin. The mutant embryos did assemble actin and myosin at the invaginating membrane fronts, but the proteins did not form circular rings, and constriction was defective.
Rings in the test tube
The in vivo experiments clearly demonstrated that septins are required for generating a curved and tightly packed ring of actin filaments. However, due to the complex molecular composition of cells it is difficult to conclude on the mechanism of ring formation. To overcome this challenge, the researchers from the research groups in Amsterdam and Paris purified septin and actin filaments, and looked at mixtures of these proteins by both fluorescence microscopy and electron microscopy. They discovered that septins are able to bundle actin filaments into tightly packed, contractile rings.
The findings provide a mechanistic basis for a number of defects during cell divison that are caused by septin depletion, such as mispositioning of the cytokinetic ring and multinucleation – in which cells form with more than one nucleus.
More information: Manos Mavrakis, Yannick Azou-Gros, Feng-Ching Tsai, José Alvarado, Aurélie Bertin, Francois Iv, Alla Kress, Sophie Brasselet, Gijsje H. Koenderink, Thomas Lecuit, Septins promote F-actin ring formation by cross-linking actin filaments into curved bundles, Nature Cell Biology, Advance Online Publication (AOP), DOI: 10.1038/ncb2921.
Journal information: Nature Cell Biology
Provided by Fundamental Research on Matter (FOM)