The science of creating catalysts for energy storage
(Phys.org) —In Inorganic Chemistry, Dr. Dan DuBois at Pacific Northwest National Laboratory shares three fundamental discoveries made to build catalysts that drive the storage of electrical energy inside chemical bonds. He was invited to write this review article after winning the American Chemical Society Inorganic Chemistry Award in 2012. DuBois was recognized for his scientific leadership in a career highlighted by outstanding science, popular seminars and talks, and a reputation as an insightful, gregarious mentor.
Replacing fossil fuels with wind and solar energy requires a method of storing the energy generated and releasing it when needed. "Both of these renewable energy sources can contribute significantly to our energy needs, but their energy output can vary over periods as short as a few minutes to as long as a year. This leads to mismatches between energy production and demand that could be overcome by energy storage," said DuBois.
One option is to store the energy in chemical bonds (i.e., fuels), and then break those bonds when the energy is needed. The necessary reactions demand an efficient catalyst based on nickel or other abundant transition metal. Designing catalysts means understanding the underlying scientific principles. With his colleagues at the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy's Office of Basic Energy Sciences, DuBois has answered fundamental questions about what makes a catalyst work.
"His work is the foundation we use to design a catalyst to do what we want to do. Without this basic knowledge, catalyst development relied largely on trial and error," said Dr. Monte Helm, Deputy Director of the Center for Molecular Electrocatalysis.
In his review, DuBois focuses on three themes: thermodynamic modeling, catalyst structure, and proton movement. Designing a catalyst involves creating a reaction path that avoids troubling intermediates with excessively high or low energy barriers. To examine intermediates, DuBois and his colleagues built comprehensive thermodynamic models to provide detailed simulations. The models include new approaches to measure thermodynamic hydride acceptor and donor abilities. They also include data about redox potentials and the solvent.
"We've used relationships extracted from the thermodynamic models to create powerful tools for predicting and understanding the relative free energies of intermediates," said DuBois.
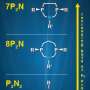
The second advance DuBois discusses is conceptually partitioning catalysts into first, second, and outer coordination spheres. The first coordination sphere is closest to the active site at the heart of the catalyst. Understanding the proton-transfer reactions that involve the second and outer spheres, once dismissed as "shrubbery," and the associated energy barriers is vital to designing needed catalysts.
Finally, the review covers the motion of protons, specifically pendant amines, small dangling molecules with strategically placed nitrogen atoms in the second coordination sphere. The amines create paths for intra- and intermolecular proton transfers. "Dan's influence on our research at PNNL is profound, as is obvious when you read this article," said Dr. Morris Bullock, Director of the Center for Molecular Electrocatalysis.
Further discoveries critical for designing the needed catalysts are being made at Pacific Northwest National Laboratory, and DuBois continues to be a part of the Center. He currently serves as an advisor, working on research and counseling other scientists.
More information: DuBois DL. 2014. "Development of Molecular Electrocatalysts for Energy Storage." Inorganic Chemistry Article ASAP. DOI: 10.1021/ic4026969
Journal information: Inorganic Chemistry
Provided by Pacific Northwest National Laboratory