Protein key to cell motility has implications for stopping cancer metastasis
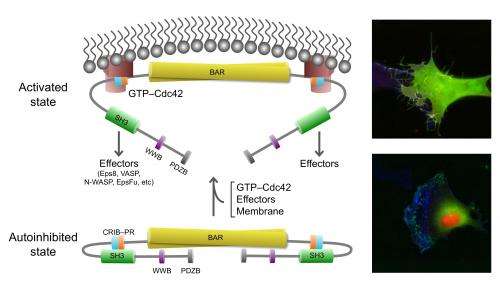
A Penn team describes how a key cell-movement protein called IRSp53 is regulated in a resting and active state, and what this means for cancer-cell metastasis. They characterized how IRSp53 connects to the cell-motility machinery by starting the formation of cell filopodia.
"Cell movement is the basic recipe of life, and all cells have the capacity to move," says Roberto Dominguez, PhD, professor of Physiology at the Perelman School of Medicine, University of Pennsylvania. Motility – albeit on a cellular spatial scale—is necessary for wound healing, clotting, fetal development, nerve connections, and the immune response, among other functions. On the other hand, cell movement can be deleterious when cancer cells break away from tumors and migrate to set up shop in other tissues during cancer metastasis.
The Dominguez team, with postdoctoral fellow David Kast, PhD, and colleagues, report online ahead of print in Nature Structural & Molecular Biology how a key cell-movement protein called IRSp53 is regulated in a resting and active state, and what this means for cancer-cell metastasis.
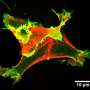
"We characterized how IRSp53 connects to the cell-motility machinery," says Kast. "It does this by starting the formation of cell filopodia - extensions that form when a cell needs to move."
"Cells move like an inchworm," explains Dominguez. "Filopodia are at the leading edge of moving cells." The trailing end of the cell follows the move forward through contraction of actin and myosin in the cytoskeleton, much like muscle contraction. A cell pushes out the leading edge of its membrane, and sticks it down on whatever it is moving across, namely other cells, and then moves the cell body along, unsticking the back end. This sets the cell up for its next move.
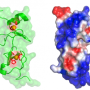
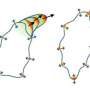
IRSp53 contains a region called a BAR domain that binds to and shapes cell membranes. Other parts of the protein connect it to the cytoskeleton (internal bits that give a cell structure and shape). Together, through the binding of cell membranes and other proteins IRSp53 regulates cell movement. The team found that in the resting state, human IRSp53 adopts a closed shape that prevents it from interacting with the membrane and the cytoskeleton. However, the binding of a signaling protein, called Cdc42, opens IRSp53, setting in motion the recruitment of a complex cellular machinery needed for motility.
One of the cytoskeleton components IRSp53 connects to is the tumor-promoting protein Eps8. IRSp53 is synergistically activated by the combined action of Cdc42 and binding of Eps8, which is upregulated in metastatic cancers.
Co-authors Tatyana Svitkina and Changsong Yang from the Penn Department of Biology, brought their expertise with living cells to the study. By introducing normal and mutant proteins into cells they could see how these proteins induced filopodia to form. The team found that mutations in critical regions of IRSp53 can either lead to enhanced or reduced filopodia formation and, as a consequence, cell motility. "This finding shows how all these different proteins converge on IRSp53 to execute precise cellular functions, and that when one factor is disrupted, other proteins are affected down the activity pathway," says Dominguez.
The team's next steps will be to screen libraries of small molecule inhibitors that interfere with the IRSp53-Eps8 interaction, to figure out how to stop unwanted cell movement before it gets too far.
Journal information: Nature Structural & Molecular Biology
Provided by University of Pennsylvania School of Medicine