March 27, 2014 feature
Of mice and molecules: In vivo photoacoustic imaging using semiconducting polymer nanoparticles
(Phys.org) —Photoacoustic imaging is a hybrid biomedical imaging modality, based on the photoacoustic effect, in which non-ionizing laser pulses are delivered into biological tissues. (More specifically, in the photoacoustic effect sound waves form due to pressure changes when a material absorbs varying-intensity modulated or pulsed light. These waves are then detected by, for example, microphones or piezoelectric sensors. The resulting photoacoustic signal is the current or voltage that provides the value indicating how the sound waves vary in time.) Recently, scientists at Stanford University developed a new class of contrast agents for photoacoustic molecular imaging – namely, near-infrared (NIR) light absorbing semiconducting polymer nanoparticles (SPNs) that produce a stronger signal than single-walled carbon nanotubes and gold nanorods – properties that allowed the researchers to perform whole-body lymph-node photoacoustic mapping on living laboratory mice. In addition, these semiconducting polymer nanoparticles possess high structural flexibility, narrow photoacoustic spectral profiles and strong resistance to photodegradation and oxidation – qualities essential to the designing the first near-infrared ratiometric photoacoustic probe for in vivo real-time imaging of the reactive oxygen species (ROS) that mediate many diseases. In short, the researchers say, their results show semiconducting polymer nanoparticles to be the perfect nanoplatform for the development of photoacoustic molecular probes.
Prof. Jianghong Rao discussed the paper that he, Dr. Kanyi Pu and their co-authors published in Nature Nanotechnology. "Firstly, there are several ideal properties a photoacoustic imaging probe should have," Rao tells Phys.org. "These are no or low toxicity, high photoacoustic efficiency, excellent photostability and chemical stability, absorption in infrared or near-infrared wavelength to avoid the tissue background light absorption and achieve better light penetration, and –for a molecular imaging probe – the ability to generate target-specific photoacoustic imaging contrast." However, Rao continues, current photoacoustic contrast agents generally do not meet all of these requirements, having either have poor photostability, poor oxidation stability, or toxicity concerns. While photoacoustic imaging promises to significantly advance molecular-level physiological and pathological visualization with deep tissue penetration and fine spatial resolution, photoacoustic molecular imaging probes must first be developed.
On the other hand, Rao notes that semiconducting polymer nanoparticles offer a number of attractive features, including being a photoacoustic imaging contrast agent, no use of toxic metals, being biologically inert, having high photostability, are resistant against oxidation, and the ability to be made with high near-infrared light absorption. "The main question," he explains, "was whether it was efficient for semiconducting polymer nanoparticles to produce acoustic signals after light excitation – and we had to examine the type of polymer to determine this. All this said, the big challenge for molecular photoacoustic imaging probes is whether they can produce a specific signal in response to their molecular targets. This requires a signal activation mechanism controlled by the molecular target."
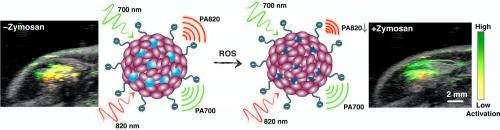
In addressing these challenges, Rao says that their key insight was that a semiconducting polymer can be formulated into a water-soluble nanoparticle and, depending on its structure, the resultant nanoparticles can be highly efficient for photoacoustic imaging. "Our key innovation in designing semiconducting polymer nanoparticles into a phootoacoustic molecular imaging probe was to introduce ratiometric imaging widely used in fluorescence imaging," he says. Ratiometric imaging techniques observe emission wavelength shifts of fluorophores (fluorescent chemical compounds that can re-emit photons upon light excitation) or by comparing the emission intensity of a fluorophore combination instead of measuring mere intensity changes. "By exciting the probe at two different wavelengths, the target activation leads to the change in the photoacoustic signal at one wavelength, so the ratio of the signals at two wavelengths will change accordingly. This allowed us to create a target-specific photoacoustic signal."
Rao describes some of the paper's interesting and important findings, starting with their fundamental demonstration that near-infrared light-absorbing semiconducting polymer nanoparticles can serve as an efficient and stable nanoplatform to allow photons to be used to generate ultrasound waves, permitting in vivo photoacoustic molecular imaging. "Semiconducting polymer nanoparticles can absorb a large amount of near-infrared light," he explains. "The absorbed energy is then dissipated as heat to generate sound waves and these waves can be detected by the ultrasound transducer and in turn exploited for photoacoustic imaging. Addressing another result – that activatable molecular imaging probes can undergo an intrinsic signal evolution upon detecting molecular targets or events, providing a real-time correlation between probe activated versus non-activated states and pathological processes on a molecular level – Rao points out that in this study, the probe produces photoacoustic signals at two different wavelengths (700 nm and 820 nm) before activation by the ROS (reactive oxygen species) molecular target. "After activation," he adds, "the signal at 820 nm is lost, and the signal at 700 nm remains. Thus this signal change reflects the presence and activity of the target. The imaging acquisition is fast, so the detection can be in real time. The imaging captures molecular change of the probe that reflects the activity of the ROS molecular target in the disease."
The paper emphasizes that full utilization of the potential of photoacoustic imaging at a depth and spatial resolution that is unattainable by fluorescence imaging requires new materials amenable to the construction of activatable photoacoustic probes. "Activatable probes can allow one to detect physiological and pathological molecular events," Rao explains. "However, most current activatable probes rely on fluorescence, which doesn't provide the deep imaging depth and high spatial resolution that photoacoustic imaging does."
Moving forward, Rao says, the scientists are continuing to explore their application for imaging – for example, photoacoustic imaging of cancer by attaching a tumor-targeting molecule to the nanoparticle. "Another area will be to explore more polymers that absorb at different near-infrared wavelength," he adds, "allowing multiple target imaging to be done simultaneously. Moreover, while this work demonstrates the imaging of reactive oxygen species, other molecular targets, such as pH and enzyme species, may be similarly imaged." Rao also points out that it may be possible for the new approach to be combined with drug delivery, effectively creating so-called theranostic nanoparticles for personalized healthcare applications by testing patients for possible reactions to a new medication, and then tailoring a treatment for them based on the test results.
Rao lists a number of applications that will emerge as a result of their research. "Our research will most likely lead to the use of semiconducting nanoparticles for photoacoustic imaging on pre-clinical animal models, such as imaging ROS in deep tissue locations in diseases," he says. "It could also lead to the development of other semiconducting polymer-based photoacoustic imaging probes, both targeting probes by conjugating a targeting ligand" (a small molecule that forms a complex with a biomolecule to serve a biological purpose) "and activatable probes signal activation by molecular targets other than ROS."
Regarding other areas of research that might benefit from their study, Rao tells Phys.org that the new nanomaterial should enhance the ability to study cancer, neurodegenerative, cardiovascular, and many other diseases in animal models, and help uncover the role of aberrant RONS (reactive oxygen and nitrogen species) in these diseases and contribute to the development of novel therapeutics. "With the translation of photoacoustic imaging to clinics," Rao concludes, "it may be applied to clinical research as well."
More information: Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice, Nature Nanotechnology 9(3), 233–239 (2014), DOI: 10.1038/nnano.2013.302
Journal information: Nature Nanotechnology
© 2014 Phys.org. All rights reserved.