Faster screening test to identify tuberculosis
With 9 million new cases and 2 million deaths annually, Tuberculosis is the second most prevalent and deadliest infectious disease worldwide. As an airborne disease, it spreads easily and is very contagious. Quick detection and identification is the key to success in preventing the spread of the disease.
Conventional tuberculosis screenings suffer from low sensitivity, specificity, and high-cost. The gold standard for the diagnosis of active tuberculosis still relies on time-consuming culture tests that take 10 to 40 days to complete the screening process.
Recent nanopore research by Assistant Professor Hung-Jen Wu of the Artie McFerrin Department of Chemical Engineering at Texas A&M University, along with the Houston Methodist Research Institute, has led to a new screening tool that quickly identifies the M. tuberculosis antigen, CFP-10.
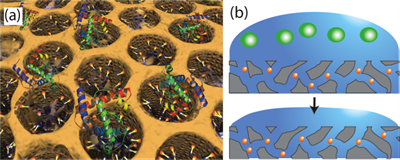
"CFP-10 is an antigen secreted by the M. tuberculosis bacteria," says Wu. "Its rod shape allows us to use various types of nanopore thin films to very easily and quickly, isolate and identify the strand using a mass spectrometer."
Wu has engineered the nanopore size, structure, and surface properties to isolate up to 90% of CFP-10 in biological samples. The whole screening process takes about nine hours including incubation time. This overall approach offers hope not only for speeding up diagnosis of active tuberculosis but also for future screening of other infectious diseases.
Provided by Texas A&M University