Combatting hospital-acquired infections with protein metal complex
Professor Yoshihito Watanabe (WPI-ITbM, Cooperating Researcher), Associate Professor Osami Shoji, Ms. Chikako Shirataki of Nagoya University and co-workers have found a new method using an artificial metalloprotein (a protein that contains a metal) to inhibit the growth of Pseudomonas aeruginosa bacteria, which is a common bacterium that can cause diseases in humans and evolves to exhibit multiple antibiotic resistance. The inhibition of growth has been achieved through the deprivation of iron uptake using an artificial metalloprotein. The study published in the online Early View on February 7, 2014 of Angewandte Chemie International Edition, is expected to bring hope in the battle against bacteria.
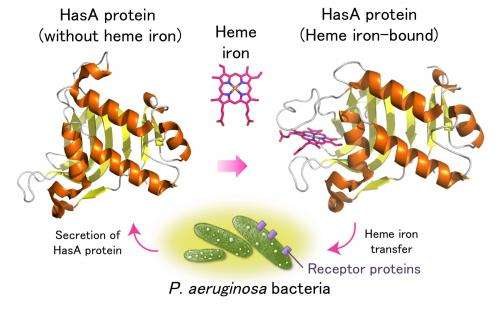
P. aeruginosa bacteria exists in many aquatic areas and is prevalent in hospitals. Although they do not usually affect healthy people, they increase the risk for infection of patients with low immunity. Their high resistance towards many antibiotics makes complete elimination of them extremely difficult. Like humans, bacteria require the uptake of heme iron for their survival, and a protein (HasA) is secreted from bacteria to capture heme from its host. The heme-bound HasA protein transfers heme via receptor proteins on the cell surface of the bacterium, P. aeruginosa (Figure 1).
"Upon looking closely at the crystal structure of the HasA protein binding heme, we considered the possibility of the HasA protein to bind to a metal complex that has a similar structure as heme" says Associate Professor Osami Shoji, who led the study. "We found synthetic metal complexes that can mimic heme and bind to the HasA protein. To our delight, one of the resulting complexes successfully inhibited growth of P. aeruginosa bacteria."
"It took us around four years to discover that phthalocyanine, which is a blue paint used on the surface of the Japanese bullet trains and road signs, could bind competitively to the HasA protein", adds Ms. Chikako Shirataki, a PhD student in her final year, "crystal structures of metal protein complexes helped us to show that the phthalocyanine-bound HasA protein blocks the receptors on the cell surface of the bacterium and thus, inhibits the uptake of heme." When bacteria are deprived of iron, further growth of the bacteria is inhibited (Figure 2).
P. aeruginosa infections can potentially lead to pneumonia and an effective treatment method is highly required. This finding by Shoji's group opens new doors to treat P. aeruginosa infections by using an unprecedented approach to inhibit the growth of bacteria. Associate Professor Shoji states, "With the advice of medical doctors, we are currently working to develop a new system to wipe out bacteria by tuning various metal complexes. Although the efficiency is not high yet, we have already established a mechanism to eliminate bacteria and we are considering how to apply it to different cases."
More information: "Inhibition of Heme Uptake in Pseudomonas aeruginosa by its Hemophore (HasAp) Bound to Synthetic Metal Complexes," Chikako Shirataki, Osami Shoji, Mitsuyoshi Terada, Shin-ichi Ozaki, Hiroshi Sugimoto, Yoshitsugu Shiro, Yoshihito Watanabe. Angewandte Chemie International Edition, February 7, 2014. DOI: 10.1002/anie.201307889
Journal information: Angewandte Chemie International Edition
Provided by ResearchSEA

















