The mechanism of caesium intercalation of graphene
Properties of many layered materials, including copper- and iron-based superconductors, topological insulators, graphite and epitaxial graphene, can be manipulated by the inclusion of different atomic and molecular species between the layers via a process known as intercalation.
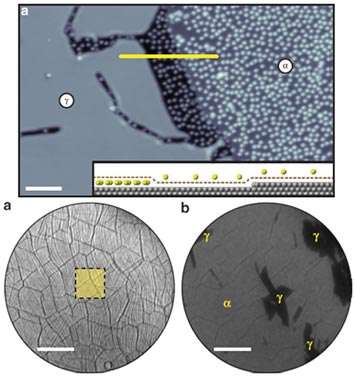
Intercalation involves complex diffusion processes along and across the layers; however, the microscopic mechanisms and dynamics of these processes are not well understood. Using in-situ microscopy to follow the intercalation process of caesium (Cs) of graphene monolayer on iridium (111) surface we discovered a novel mechanism for intercalation and entrapment of alkali atoms under epitaxial graphene. We find that the intercalation is adjusted by the van der Waals interaction, with the dynamics governed by defects anchored to graphene wrinkles.
There are many potentially useful properties associated with the intercalation of epitaxial graphene systems. For instance, it was demonstrated that by precise control of the intercalation interface, laterally well-defined mesoscopic regions of n- and p-doped graphene—that is, p–n graphene junctions, can be formed. It was also demonstrated that it is possible to form well-defined ferromagnetic nano-islands under graphene. Therefore, it is extremely important to understand in detail how the properties of chemically modified graphene depend on its chemical environment. From the view point of chemical kinetics, understanding the penetration and diffusion of ions under graphene sheets in atomistic detail is of fundamental importance for the design of novel batteries and supercapacitors.
What Are The Specifics?
- CFN Capabilities: CFN's Elmitec III PEEM/LEEM endstation at the NSLS beamline U5UA was used to characterize adsorption and intercalation of caesium (Cs) at the nanoscale.
- The diversity and sensitivity of the physical and chemical properties of graphene and other layered systems, with respect to intercalation of charge-donating species, are directly related to prominent effects, such as super-conductivity in graphite. From the view point of chemical kinetics, understanding the penetration and diffusion of ions under graphene sheets in atomistic detail is of fundamental importance for the design of novel batteries and supercapacitors.
More information: The mechanism of caesium intercalation of graphene. M. Petrović, et al. Nature Communications 4, Article number: 2772 DOI: 10.1038/ncomms3772 . Received 15 April 2013 Accepted 15 October 2013 Published 11 November 2013
Journal information: Nature Communications
Provided by Brookhaven National Laboratory