Toward lowering titanium's cost and environmental footprint for lightweight products
A novel method for extracting titanium, a metal highly valued for its light weight, high strength, corrosion resistance and biocompatibility, could lower its cost and make it more widely accessible, for example, for producing lighter car parts to improve fuel efficiency. The method, which significantly reduces the energy required to separate it from its tightly bound companion, oxygen, appears in the Journal of the American Chemical Society.
Zhigang Zak Fang and colleagues note that while titanium is the fourth most common metal in the Earth's crust, the high-energy, high-cost method used to extract it prevents its use in broader applications. The metal's light weight, strength, stability and corrosion resistance earned it valued roles on the Mars Odyssey mission, in wedding rings and in deep-sea submersibles. Titanium also could be used to significantly lighten and strengthen commercial products and materials. But currently, titanium is too expensive for widespread use. The most common technique, called the Kroll process, used to extract the metal from titanium oxide was invented in the 1930s and has undergone slight improvements. But by and large, the method, which requires temperatures over 1,800 degrees Fahrenheit, keeps prices for the metal high. Fang's team decided to try out a new approach to make titanium more accessible.
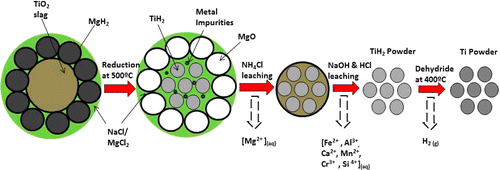
The scientists discovered that they could eliminate the energy-intensive steps of the Kroll process. In the lab, they successfully tested a new series of reactions for isolating titanium that halves the temperature requirements of the conventional method and consumes 60 percent less energy.
More information: "A New, Energy-Efficient Chemical Pathway for Extracting Ti Metal From Ti Minerals" J. Am. Chem. Soc., 2013, 135 (49), pp 18248–18251. DOI: 10.1021/ja408118x
Abstract
Titanium is the ninth most abundant element, fourth among common metals, in the Earth's crust. Apart from some high-value applications in, e.g., the aerospace, biomedicine, and defense industries, the use of titanium in industrial or civilian applications has been extremely limited because of its high embodied energy and high cost. However, employing titanium would significantly reduce energy consumption of mechanical systems such as civilian transportation vehicles, which would have a profound impact on the sustainability of a global economy and the society of the future. The root cause of the high cost of titanium is its very strong affinity for oxygen. Conventional methods for Ti extraction involve several energy-intensive processes, including upgrading ilmenite ore to Ti-slag and then to synthetic rutile, high-temperature carbo-chlorination to produce TiCl4, and batch reduction of TiCl4 using Mg or Na (Kroll or Hunter process). This Communication describes a novel chemical pathway for extracting titanium metal from the upgraded titanium minerals (Ti-slag) with 60% less energy consumption than conventional methods. The new method involves direct reduction of Ti-slag using magnesium hydride, forming titanium hydride, which is subsequently purified by a series of chemical leaching steps. By directly reducing Ti-slag in the first step, Ti is chemically separated from impurities without using high-temperature processes.
Journal information: Journal of the American Chemical Society
Provided by American Chemical Society



















