Exposing the secret pathways behind photosynthesis
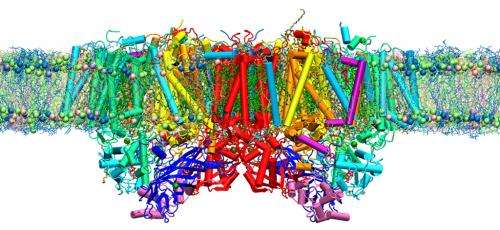
New insights into the behavior of photosynthetic proteins from atomic simulations could hasten the development of artificial light-gathering machines.
The protein complex known as photosystem II splits water molecules to release oxygen using sunlight and relatively simple biological building blocks. Although water can also be split artificially using an electrical voltage and a precious metal catalyst, researchers continue to strive to mimic the efficient natural process. So far, however, these efforts have been hampered by an incomplete understanding of the water oxidation mechanism of photosystem II. Shinichiro Nakamura from the RIKEN Innovation Center and colleagues have now used simulations to reveal the hidden pathways of water molecules inside photosystem II.
At the heart of photosystem II is a cluster of manganese, calcium and oxygen atoms, known as the oxygen-evolving complex (OEC), that catalyzes the water-splitting reaction. Recent high-resolution x-ray crystallography studies have revealed the precise positions of the atoms in the OEC and of the protein residues that contact the site. While this information has yielded important structural clues into photosynthetic water oxidation, the movements of water, oxygen and protons within the protein complex are still the subject of much speculation.
To resolve this problem, researchers have turned to molecular dynamics (MD) simulation, a technique that models the time-dependent behavior of biomolecules using thermodynamics and the physics of motion. While previous MD simulations of photosystem II have involved the use of approximate models that focus only on protein monomers or the main OEC components, Nakamura's team took a different approach. "Our hypothesis was that we cannot understand the mechanism of oxygen evolution just by looking at the manganese-based reaction center," he says. "Therefore, we carried out a total MD simulation, without any truncation of the protein or simplification."
In their simulation, the team embedded an exact model of photosystem II inside a thylakoid—a lipid and fatty-acid membrane-bound compartment found in the chloroplasts of plant cells. After initial computations confirmed the reliability of their model, the researchers performed a rigorous MD simulation of the protein—membrane system in the presence of more than 300,000 water molecules (Fig. 1). "The results indicated that water, oxygen and protons move through photosystem II not randomly but via distinct pathways that are not obviously visible," says Nakamura.
The pathways revealed by the simulations are delicately coupled to the dynamic motions of the photosystem II protein residues. While such intricate activity is currently impossible to reproduce artificially, the researchers suspect that combining quantum-chemical calculations with MD simulations could help to unlock the mysterious principles behind the highly efficient oxygen-evolution reactions of this remarkable biological factory.
More information: Ogata, K., Yuki, T., Hatakeyama, M., Uchida, W. & Nakamura, S. All-atom molecular dynamics simulation of photosystem II embedded in thylakoid membrane. Journal of the American Chemical Society 135, 15670–15673 (2013). dx.doi.org/10.1021/ja404317d
Journal information: Journal of the American Chemical Society
Provided by RIKEN


















