Nickel segregation, cation spatial distribution and tightly integrated phases occur in pristine battery material
(Phys.org) —To prevent fading in a layered lithium cathode that holds promise for heavy duty transportation use, scientists at Pacific Northwest National Laboratory, FEI Company, and Argonne National Laboratory obtained a definitive view of a pristine cathode made of lithium, nickel, manganese, and oxygen. The cathode is known as Li1.2Ni0.2Mn0.6O2 or LMNO. Controversy has encircled this material. Some state it's a solid solution; others, a composite. To address this debate, the team used a suite of instruments and determined the material is a composite with tightly integrated phases where the surface contains higher concentrations of nickel and low concentrations of oxygen and electron-rich manganese.
"If we want to improve the cycle life and capacity of the layered cathode, we must have this type of clarity around the atomic structure and possible cation ordering," said Dr. Nigel Browning, the Chief Science Officer of PNNL's Chemical Imaging Initiative and a microscopy expert who worked on the study.
Replacing gasoline-powered cars with electric-powered ones could drop U.S. reliance on oil imports by up to 60%, and reduce harmful emissions as much as 45%, depending on the technological mix used. The key is long-lasting, energy-dense batteries. Innovative LMNO cathodes possess high voltage and high specific capacity. Yet, the material is far from ideal. Capacity and voltage fading issues are linked to the cathode's structure during charging and discharging. The team's characterization research provides the foundation necessary for needed discoveries.
"The ever-growing energy demand of information and transportation relies on lithium-ion batteries for power storage, because of their relatively high energy density and design flexibility. We need it better and we need it now, which contributes to the main driving force for creating new materials for energy storage," said Dr. Chongmin Wang, chemical imaging expert at PNNL and lead investigator on this study.
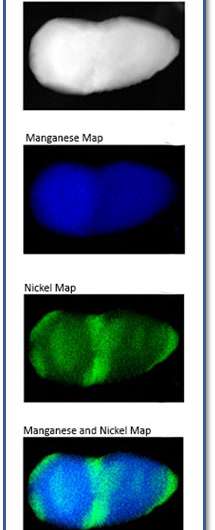
Using a combination of aberration-corrected scanning transmission electron microscopy, X-ray energy-dispersive spectroscopy, electron energy loss spectroscopy, and complementary multi-slice image simulation, the team probed Li1.2Ni0.2Mn0.6O2 nanoparticles. On the particle's surface, they made several discoveries. A surface with a unique structural characteristic is prone to contain a higher concentration of nickel atoms than the core of the particle, while manganese atoms are more prevalent at the core than the surface. Oxygen vacancies on the particle's surface result in manganese atoms having a valence state or electron configuration of +2.2 on the surface, while the manganese at the particle's center is +4.0.
"This finding indicates a big variation in the local stoichiometry," said Dr. Jun Liu, a materials expert who worked on this study and who is also Director of PNNL's Energy Processes and Materials Division.
Finally, each particle contains both of the material's parent phases. The lattice parameter and crystal structure similarity of the layered LiMO2 phase and the layered Li2MO3 phase allow for the structural integration.
"This detailed characterization allowed us to gain a more complete picture of the material," said Wang. "Clarification of the material's structure—nanoscale phase separation, cation ordering and oxygen vacancy formation—will undoubtedly shine a new light on probing how the material behaves during battery performance and will inspire us to improve its functionality via controlled synthesis."
The team is now working to understand how the material evolves during charge/discharge cycles.
More information: Gu M, A Genc, I Belharouak, D Wang, K Amine, S Thevuthasan, DR Baer, JG Zhang, ND Browning, J Liu, and C Wang. 2013. "Nanoscale Phase Separation, Cation Ordering, and Surface Chemistry in Pristine Li1.2Ni0.2Mn0.6O2 for Li-Ion Batteries." Chemistry of Materials 25(11):2319-2326. DOI: 10.1021/cm4009392
Journal information: Chemistry of Materials
Provided by Environmental Molecular Sciences Laboratory