Five-dimensional crystallography probes molecular structure
(Phys.org) —Successful development of new pharmaceuticals could be the payoff from five-dimensional crystallography, a new experimental technique employed by researchers carrying out studies at the BioCARS facility at the U.S. Department of Energy Office of Science's Advanced Photon Source (APS).
X-ray crystallography uses x-rays to investigate protein structure, and has played an increasingly important role in drug discovery in recent decades. This technique essentially represents a type of extremely high-resolution microscopy for investigating the molecular structure of proteins with near-atomic resolution. This facilitates improved understanding of their function, and provides vital structural information on specific protein targets. In this way it can contribute to the design of new drugs that target specific proteins, or to the engineering of enzymes for specific industrial processes.
Protein folding is the process by which a protein takes on a specific three-dimensional (3-D) structure essential for it to function. Many proteins, called enzymes, promote or catalyze specific chemical reactions. The 3-D structures of these proteins change during the course of the reactions. Free energy landscapes represent multidimensional hyper-surfaces that determine the progress of catalyzed reactions, and characterization of these landscapes allows the reaction to be described and visualized.
In time-resolved crystallography (TRX), x-ray diffraction by crystals is utilized to examine in real time the structures of proteins as they are changing and therefore improve understanding of their function and gain important structural information on specific protein targets.
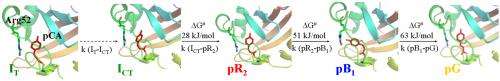
Five-dimensional (5-D) crystallography is a specific form of the TRX technique where, in addition to space and time, temperature is a variable as well. It allows complete characterization of all features of a chemical reaction, including the structure of its intermediate compounds, as well as the reaction kinetics, and barriers of activation between the intermediates. It provides an essential and direct link between the structural changes and energy changes in the chemical reaction.
In this study, Marius Schmidt (University of Wisconsin-Milwaukee) and colleagues from the University of Wisconsin-Milwaukee, The University of Chicago, The Institute for Basic Science (Republic of Korea), and Korea Advanced Institute of Science and Technology (Republic of Korea) employed the photocycle reaction of photoactive yellow protein (PYP), a bacterial photosensor protein, as a model for 5-D crystallography. In this reaction, a sequence of light-induced structural changes in the protein produces distinct intermediate structures.
Using data sets collected from TRX experiments conducted on crystals of PYP at the BioCARS 14-ID beamline at the Argonne National Laboratory APS, the researchers investigated the effect of changing temperature on the kinetics of inter-conversion between intermediates formed during the photocycle. By lowering the temperature below 0o C, the rate of the chemical reactions slowed down, allowing an early intermediate like IT to be observed on the nanosecond time-scale (see figure), whereas it had previously only been evident using picosecond TRX.
The study also showed that from -40º C to +50º C, the reaction proceeded in a temperature-dependent manner. However, above 50º C, the optimum temperature for the reaction was exceeded, and its rate slowed down again. Most importantly, such temperature-dependent data allowed energies of activation between intermediates to be determined directly from x-ray data.
"Using the BioCARS beamline, time-resolved high-resolution Laue crystallographic data with 100-picosecond time resolution can be collected and analyzed swiftly with novel data collection and data processing strategies. Without this beamline and the support of the BioCARS staff, these experiments would have been impossible," Schmidt said.
Data from this study showed how 5-D crystallography may demonstrate energy changes associated with barriers of activation in the photocycle reaction of PYP. The results will be important in guiding future work to investigate changing energy landscapes of other enzymatic reactions, and may contribute to the development of novel drugs that target a specific protein.
Provided by Argonne National Laboratory