More transparency: Optically transparent water oxidation catalyst made from copper nanowires
(Phys.org) —Hydrogen is used as an energy source in fuel cells and can be produced from water by using sunlight and a suitable catalyst. In the journal Angewandte Chemie, American researchers have now introduced a new electrocatalyst consisting of a conductive network of core-shell nanowires that is just as efficient as conventional metal oxide films on indium tin oxide (ITO) and a great deal more transparent and robust.
Nickel and cobalt oxides are attractive anode materials for the oxidation of water because they are readily available and demonstrate high catalytic activity. For use in photoelectric synthesis cells, in which chemical conversions are driven by light, the oxides are typically electrodeposited onto ITO substrates. ITO is used because of its high transmittance and low sheet resistance. However, the high potentials required for the oxidation of water cause the conductivity of ITO surfaces to fall. In addition, indium is expensive and the production of ITO films is costly. Another disadvantage is that the catalytic oxide layers reduce the light transmittance and thus the light captured by the photovoltaic components.
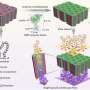
A team led by Benjamin J. Wiley at Duke University in Durham has now developed a new approach to solve these problems. Their trick is to replace the ITO electrode with a conductive network of copper nanowires. Copper is a common element and is orders of magnitude cheaper than indium. In addition, the nanowires can be quickly, easily, and inexpensively deposited onto a glass surface from a liquid. Afterward, the researchers electrolytically deposit nickel or cobalt onto the nanowires. The resulting network of core-shell nanowires is as efficient as metal oxide films of similar composition for the electrocatalytic oxidation of water, but is several times more transparent.
The nanowire film can also be deposited onto a flexible sheet of polyethylene terephthalate (PET) plastic instead of glass. Unlike ITO-based electrocatalysts on PET substrates, which suffer from significant loss of conductivity after repeated bending, the film made of nanowires isn't really affected. The scientists are optimistic that their approach will open up new possibilities for the design of more efficient, mechanically robust, and affordable light-harvesting systems for the production of solar fuels.
More information: Benjamin J. Wiley, Optically Transparent Water Oxidation Catalysts Based on Copper Nanowires, Angewandte Chemie International Edition, Permalink to the article: dx.doi.org/10.1002/anie.201306585
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley