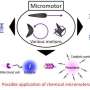
Stirred from within: Micromotors mix for more effective oxidative degradation of chemical weapons
(Phys.org) —Rapidly and efficiently converting chemical weapons into nontoxic products in remote areas is one of the most difficult tasks in the disposal of weapons of mass destruction. In the journal Angewandte Chemie, a team from the University of California, San Diego has now described how self-propelled micromotors can accelerate the oxidative neutralization of nerve agents by intensively mixing the remediation solution.
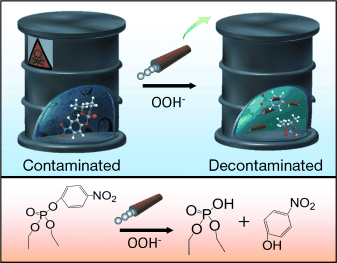
Environmentally friendly processes that use hydrogen peroxide and an activator (e.g. sodium bicarbonate) to degrade chemical weapons like sarin, VX, soman, and mustard gas have recently replaced earlier chlorine-based methods. However, they generally require high concentrations of peroxide, long reaction times, and intensive mechanical mixing—which can be extremely problematic in the elimination of stocks of chemical weapons in remote regions or enemy camps.
A team headed by Joseph Wang has now developed a novel strategy based on mixing of the remediation solution with self-propelled micromotors. The motors are tiny conical tubes made from a bilayer with a polymer on the outside and platinum on the inside. In this process, hydrogen peroxide acts as both the oxidizing agent for the chemical weapons and fuel for the micromotors. As the hydrogen peroxide is catalytically decomposed on the inner platinum surface, oxygen bubbles are formed. The bubbles exit the tubes at their rear (wider) end, pushing them through the liquid. The movement of the motors through the liquid combined with the gas bubbles provides for efficient mixing of the remediation solution. This significantly increases both the turnover and the speed of the decontamination reaction without requiring high concentrations of peroxide.
Wang's team was able to demonstrate the efficiency of their new method by breaking down a variety of organophosphate pesticides with chemical structures similar to those of organophosphate nerve agents. In a demonstration reaction, 1.5 million micromotors in a volume of about 15 mL achieved mixing comparable to a magnetic stirrer at 200 revolutions per minute.
The concept of mixing through the movement of self-propelled micromotors is not limited to the neutralization of chemical weapons. It could also be used to accelerate chemical reactions in general. This could be useful in applications like microreactors, where mechanical mixing is often difficult.
More information: Wang, J. Micromotor-Based High-Yielding Fast Oxidative Detoxification of Chemical Threats, Angewandte Chemie International Edition. dx.doi.org/10.1002/anie.201308072
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie