The key to the treasure in wood

(Phys.org) —In future, it could be easier to break down wood, as a source of raw materials, into its constituent parts. Chemists at the Max Planck Institut für Kohlenforschung in Mülheim an der Ruhr have found an efficient way of making the components of the biopolymer lignin easier to use. Lignin stabilises plant cells and contains organic compounds, which are valuable to the chemicals industry for the production of biofuels, for example. The compounds in lignin are, however, difficult to access. The chemists in Mülheim can now chemically convert these building blocks so that they are more readily available.
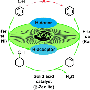
We are all aware of how finite fossil fuels are when we buy petrol or heating oil: fuel prices are constantly rising. Researchers at the Max Planck Institut für Kohlenforschung in Mülheim an der Ruhr are therefore searching for sustainable and climate-neutral alternatives to fossil fuels – not only for tomorrow's petrol, but also as starting substances in the chemicals industry. Lignin could be a source for such materials. Lignin is a biopolymer, stored by trees and shrubs in their cell walls. It penetrates the cellulose fibres of wood cells, making them rigid – and woody. The highly cross-linked chain molecules constitute 20 to 30 percent of the dry mass of woody plants; their building blocks could be useful in the chemicals industry, for example when refining biofuels or as starting materials for plastics.
"We have known about the potential of lignin for a very long time," explains Roberto Rinaldi, Max Planck Research Group Leader at the Mülheim-based Institute. Up until now, however, the treasure in the wood could not be extracted – at least not in a cost-effective way. While chemists have been able to break down the tightly cross-linked chain molecules in lignin into smaller units using an acid at high temperatures, the result has been an unruly mixture of countless compounds containing oxygen, which are difficult to separate. Brazilian researcher Rinaldi and his Group have now helped to solve this problem. The chemists have found a relatively simple way of cleaving lignin and simultaneously removing as much oxygen as possible from these compounds. The remaining compounds are primarily hydrocarbons, mostly arenes, which are aromatic compounds that are easier to sort.
Three reactions can be combined, because two catalysts interact
The chemical mix of the cleaved lignin consists of phenols and other aromatic compounds containing oxygen. The researchers found the key to the chemical treasure trove in wood when they allowed the three reactions, needed to convert the lignin building blocks into oxygen-free arenes, to take place at the same time in one pot. In the interlinked reactions, a simple alcohol extracts oxygen from the phenols in a series of intermediate steps. In this process, some of the starting materials are temporarily hydrated, i.e. supplied with hydrogen.
"We can combine the reactions because we allow two catalysts to interact," explains Roberto Rinaldi. Catalysts are chemical tools, which initiate or accelerate reactions but remain chemically unchanged themselves at the end of the reaction. The catalysts used by Rinaldi are not particularly exotic: one of them is Raney nickel, a powder containing mainly porous nickel that hydrogenates organic molecules; zeolites, porous aluminosilicate minerals that extract water from an intermediary product of the chemical 'triple jump', are also used. "These are not newly invented catalysts," says Rinaldi. "We are just approaching the problem of making lignin usable with new methods."
The combined process needs less energy
Up until now, high temperatures of up to 500 degrees Celsius and pressures of 200 bar (200 times atmospheric pressure) have been necessary to fragment the lignin structure. In contrast, the combined process used by the researchers in Mülheim takes place in relatively mild conditions – at around 150 degrees and at pressures of less than 40 bar – and therefore requires less energy. "Building the apparatus needed for the reactions should not be a complicated procedure," says Rinaldi.
And even if the yields of the individual substances extracted from the lignin are insufficient for industrial purposes, the products are at least suitable for refining synthetic plastics. This is because they are very rich in energy and do not have to be available in their pure form for fuels. "The fuels that are manufactured using the Fischer-Tropsch process do not contain such hydrocarbons, but are needed for aviation and petrol engines."
As Roberto Rinaldi and his colleagues are convinced that their discovery will be of practical significance, they have patented their procedure. They published their results in the journal Angewandte Chemie as a "Hot Paper".
More information:
Wang, X. and Rinaldi, R. A Route for Lignin and Bio-Oil Conversion: Dehydroxylation of Phenols into Arenes by Catalytic Tandem Reactions,
Angewandte Chemie int. ed.,12 September 2013. DOI: 10.1002/anie.201304776
Journal information: Angewandte Chemie
Provided by Max Planck Society